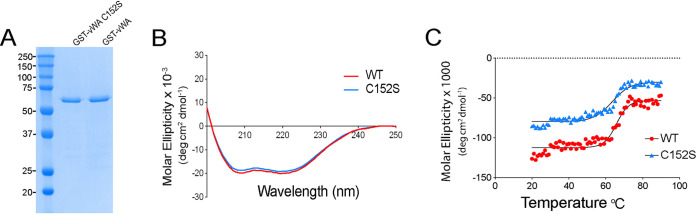
FIG 3.
vWA-C152S mutation does not substantially alter conformation of the vWA domain in solution. (A) Coomassie blue-stained SDS-PAGE of ∼1 μg of purified wild-type GST-vWA domain and GST–vWA-C152S fusion proteins expressed from a pGEX plasmid backbone and purified from E. coli BL21(DE3) cells as detailed in Materials and Methods. The molecular weight markers are indicated. (B) Far-UV circular dichroism (CD) spectra shown in molar ellipticity for the WT GST-vWA domain (red line) and GST–vWA-C152S mutant (blue line) between 195 and 250 nm at 20°C. (C) Curves of ellipticity at a 208-nm wavelength as a function of temperature for the WT and mutant fusion proteins. Spectra were recorded for each sample from 20 to 90°C in 1° increments. Curves were fitted to a Boltzmann sigmoidal equation, and the V50 value (mid-point of the slope) was determined (65.8 versus 63.5°C for the GST-WT vWA domain and GST–vWA-C152S fusion variant, respectively).