Abstract
Background
We aimed to investigate the characteristics and pretreatment risk factors for postoperative pulmonary complications (PPCs) after neoadjuvant concurrent chemoradiotherapy (CRTx) in patients with non‐small cell lung cancer (NSCLC).
Methods
We retrospectively reviewed data of 122 patients who underwent curative resection after neoadjuvant CRTx for NSCLC between 2007 and December 2019. Clinical data, including pulmonary function and body mass index (BMI) at the time of concurrent CRTx initiation, were analyzed. We performed logistic regression analyses to identify the risk factors for PPCs and built a nomogram with significant factors.
Results
Of the 122 patients included (mean age, 60.1 ± 9.7 years; 69.7% male), 27 experienced PPCs (severity grade ≥ 2). The most common PPCs were pneumonia (n = 17). Patients with PPCs had a significantly longer hospital stay (median 6.0 vs. 17 days, p < 0.001) and a higher in‐hospital mortality rate (1.1% vs. 29.6%, p < 0.001). In multivariable analysis, lower BMI (odds ratio [OR] 0.796, 95% confidence interval [CI] 0.628–0.987, p = 0.038), no comorbidity (OR 0.220, 95% CI: 0.059–0.819, p = 0.048), smoking history (OR 4.362, 95% CI: 1.210–15.720, p = 0.024), and %predicted DLCO <60% (OR 3.727, 95% CI: 1.319–10.530, p = 0.013) were independent risk factors for PPCs. The predictive accuracy of the nomogram built with factors was excellent (concordance index: 0.756).
Conclusions
The nomogram constructed with factors identified in multivariable analysis could serve as a reliable tool for evaluating the risk of PPCs in the patients who underwent neoadjuvant CRTx for NSCLC.
Keywords: chemoradiotherapy, neoadjuvant therapy, nomogram, non‐small cell lung carcinoma
Nomogram predicting the risk of postoperative pulmonary complications in the patients who underwent neoadjuvant concurrent chemoradiation treatment for non‐small cell lung cancer could serve as a reliable tool for individualized treatments.

INTRODUCTION
Existing treatment for stage IIIA/N2 non‐small cell lung cancer (NSCLC) is controversial because of its heterogeneity. 1 Neoadjuvant preoperative treatment followed by surgical resection is a possible treatment strategy for these patients. 2 Neoadjuvant chemotherapy (CTx) has potential benefits in reducing tumor size and increasing the possibility of complete resection while also eliminating micrometastatic disease,3 and adding preoperative radiotherapy (RTx) to CTx may provide advantages over chemotherapy alone by increasing local control, response rate, and downstaging the tumor. 4 Multiple studies, including randomized trials and meta‐analyses, have illustrated the wide range of oncological outcomes of surgical resection after neoadjuvant treatment. Several studies reported that patients who achieved mediastinal downstaging after neoadjuvant treatment may benefit from surgical resection, especially lobectomy, 3 , 5 , 6 whereas those with persistent residual disease also showed prognostic improvement after surgery. 7 , 8 Because the oncological outcome is controversial, proper selection of candidates for surgical resection is essential, and valid assessment of surgical risks in these patients is crucial.
Lung cancer surgery carries a considerable risk of postoperative pulmonary complications (PPCs), which are the major causes of morbidity and mortality after this procedure. 9 Neoadjuvant treatment (CTx or CRTx) was reported as a conceivable risk factor for PPCs in several studies. 10 , 11 , 12 However, only a few studies have investigated the incidence and risk factors for PPCs after neoadjuvant concurrent CRTx for NSCLC. 13 , 14 Moreover, predictive or discriminative factors, including neoadjuvant CRTx following surgery, for assigning patients with advanced NSCLC to multimodality treatment are still not established. Therefore, this study aimed to investigate the incidence and risk factors of PPCs after neoadjuvant concurrent CRTx and to construct a nomogram to help identify the subset of patients at an increased risk of PPCs.
METHODS
Patients
This single‐institution retrospective study was conducted at the Yonsei University College of Medicine with the approval of its Institutional Review Board (4‐2019‐1244). The requirement for informed consent was waived. By reviewing electronic medical records, we identified patients diagnosed with clinical stage IIIA NSCLC according to the eighth edition of the American Joint Committee on Cancer staging system. Patients who received neoadjuvant concurrent CRTx and curative resection for NSCLC between January 2007 and December 2019 were included. Patients who underwent salvage surgery were excluded from the study. At our institute, the preferred treatments for patients with potentially resectable stage IIIA are neoadjuvant concurrent CRTx. Additionally, patients with chest wall invasion or Pancoast tumors were considered candidates for neoadjuvant CRTx. The decision to initiate neoadjuvant CRTx and proceed with surgical resection was made after a multidisciplinary approach. We assessed patients with the European Cooperative Oncology Group (ECOG) scale and selected the patients with ECOG 0 to 1. If the patients showed marginal performance status, we performed additional presurgical evaluation such as cardiopulmonary exercise (CPX) testing.
The neoadjuvant CRTx protocols followed the standard regimen including weekly standard chemotherapy with five or six cycles of docetaxel plus cisplatin or paclitaxel plus carboplatin and radiation therapy (dose 40–50 Gy).
Data collection
Patient demographic and clinical characteristics included age, sex, body mass index (BMI), smoking history, age‐adjusted Charlson Comorbidity Index (CCI), 15 pulmonary function test, tumor histology, clinical and pathological stage, and type of surgery. BMI and pulmonary function tests were performed before CRTx. Cutoff values for BMI were based on the World Health Organization guidelines for the Asia‐Pacific region. 16 Clinical and pathological staging was reclassified according to the eighth edition of the TNM classification. 17
PPCs were defined as complications that were more than grade 2 according to the Clavien‐Dindo classification that occurred during hospitalization or readmission up to 30 days postoperatively 18 and included pneumonia/acute respiratory distress syndrome (ARDS), atelectasis requiring bronchoscopy, bronchopleural fistula, empyema, prolonged air leak lasting more than 10 days, and pneumothorax. The definitions of each complication were in accordance with the joint standardization of the Society of Thoracic Surgery and the European Society of Thoracic Surgeons. 19
Statistical analysis
Statistical analysis was conducted using SPSS software (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, version 23.0. Armonk, NY), R version 3.5.1 (R Foundation for Statistical Computing), and SAS version 9.4 (SAS Institute Inc.). Continuous variables were compared using Student's t‐test or the Mann–Whitney U test. Categorical variables were compared using the chi‐square test or Fisher's exact test. We conducted univariable and multivariable logistic regression analyses to identify pretreatment predictors of PPCs. Pretreatment variables with a p‐value ≤0.05 were included in the multivariable analysis. All tests were two‐sided, and p < 0.05. We built a nomogram model based on the results of the multivariable analysis. 20 The predictive ability of the nomogram was evaluated using the concordance index (C‐index) and bootstrapping to illustrate a calibration curve. The value of the C‐index ranges from 0.5 to 1.0, and a larger C‐index is consistent with a more accurate discriminative abnormality. Two hundred bootstrap resamples were used for the internal validation. A calibration curve was generated by plotting the nomogram‐predicted probability against the actual outcome, and in a well‐calibrated model, the value was expected to be approximately 45°.
RESULTS
Baseline characteristics of patients
During the study period, 5029 consecutive patients underwent curative resection for NSCLC. Among them, 122 patients received concurrent neoadjuvant CRTx of whom 27 (22.1%) had PPCs. The patient demographic characteristics are described in Table 1.
TABLE 1.
Baseline patient characteristics with and without postoperative pulmonary complications
| Total | With PPCs | Without PPCs | p value | |
|---|---|---|---|---|
| (n = 122) | (n = 27) | (n = 95) | ||
| Age (years, SD) | 60.1 ± 9.7 | 62.4 ± 10.3 | 59.5 ± 9.5 | 0.170 |
| Male (n, %) | 85 (69.7%) | 23 (85.2%) | 62 (65.3%) | 0.080 |
| Smoking (n, %) | 83 (68.0%) | 24 (88.9%) | 59 (62.1%) | 0.030 |
| Preoperative comorbidity (n, %) | 56 (45.9%) | 17 (63.0%) | 39 (41.1%) | 0.072 |
| Pulmonary TBc (n, %) | 11 (9.0%) | 5 (18.5%) | 6 (6.3%) | 0.064 |
| Age‐adjusted CCI [IQR] | 4 [3;5] | 5 [4;5] | 4 [3;5] | 0.006 |
| BMI (kg/m2, SD) | 24.2 ± 3.4 | 22.3 ± 3.6 | 24.8 ± 3.1 | 0.001 |
| FEV1 (L, SD) | 2.4 ± 0.6 | 2.1 ± 0.5 | 2.4 ± 0.6 | 0.014 |
| FEV1 (%predicted, SD) | 92.7 ± 17.0 | 95.0 ± 16.4 | 84.0 ± 16.9 | 0.006 |
| DLCO (mL/min per mmHg, SD) | 17.9 ± 4.5 | 14.4 ± 3.6 | 18.9 ± 4.3 | 0.001 |
| DLCO (%predicted, SD) | 96.2 ± 20.1 | 98.8 ± 18.2 | 86.9 ± 24.0 | 0.011 |
| Histology (n, %) | 0.425 | |||
| Adenocarcinoma | 64 (52.5%) | 13 (48.1%) | 51 (53.7%) | |
| Squamous | 54 (44.3%) | 14 (51.9%) | 40 (42.1%) | |
| Other | 4 (3.3%) | 0 (0.0%) | 4 (4.2%) | |
| Clinical N2 (n, %) | 94 (77.0%) | 22 (81.5%) | 72 (75.8%) | 0.718 |
Note: Data are presented as mean ± standard deviation, number (%), or mean (range).
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; CTx, chemotherapy; DLCO, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in 1s; IQR, interquartile range; PPC, postoperative pulmonary complication; SD, standard deviation; TBc, tuberculosis.
The mean age of the patients was 60.1 ± 9.7 years, and 85 (69.7%) were male. A higher proportion of patients with a smoking history had PPCs than those without a smoking history (88.9% vs. 62.1%, p = 0.030). A higher proportion of the patients with PPCs had preoperative comorbidities (63.0% vs. 41.1%, p = 0.044). The patients with PPCs had higher age‐adjusted CCI (5 [interquartile range, IQR, 4–5] vs. 4 [IQR, 3–5], p = 0.006) and lower BMI (22.3 ± 3.6 vs. 24.8 ± 3.1, p = 0.001) than those without PPCs. Patients with PPCs had poorer lung function than those without PPCs (FEV1 [% predicted), 95.0 ± 16.4 vs. 84.0 ± 16.9, p = 0.006; lower diffusing capacity for carbon monoxide [DLCO] [%predicted], 98.8 ± 18.2 vs. 86.9 ± 24.0, p = 0.011).
Operative and postoperative outcomes
Operative details and postoperative outcomes are described in Table 2. The total operation time was significantly shorter in patients without PPCs (174.0 ± 59.9 vs. 210.4 ± 70.9, p = 0.009). Lobectomy was the most frequently performed operation (96 patients; 78.7%) followed by pneumonectomy (14 patients; 11.5%). Twenty‐five patients (20.5%) showed complete pathological remission. Pleural seeding was found in one patient during surgery and was reclassified as pathologic stage 4a, and that patient underwent lobectomy and partial pleurectomy.
TABLE 2.
Operative and postoperative outcomes in the patients according to postoperative pulmonary complications
| Total | With PPC | Without PPC | p value | |
|---|---|---|---|---|
| (n = 122) | (n = 27) | (n = 95) | ||
| VATS (n, %) | 44 (36.1%) | 8 (29.6%) | 36 (37.9%) | 0.547 |
| Total operation time (min, SD) | 182.1 ± 64.0 | 210.4 ± 70.9 | 174.0 ± 59.9 | 0.009 |
| Estimated blood loss (ml, IQR) | 150 (50;350) | 250 (100;385) | 150 (50;300) | 0.067 |
| Surgical extent (n, %) | 0.469 | |||
| Lobectomy | 96 (78.7%) | 19 (70.4%) | 77 (81.1%) | |
| Bilobectomy | 12 (9.8%) | 4 (14.8%) | 8 (8.4%) | |
| Pneumonectomy | 14 (11.5%) | 4 (14.8%) | 10 (10.5%) | |
| ypT (n, %) | 0.726 | |||
| 0 | 33 (27.0%) | 8 (29.6%) | 25 (26.3%) | |
| 1 | 47 (38.5%) | 9 (33.3%) | 38 (40.0%) | |
| 2 | 23 (18.9%) | 4 (14.8%) | 19 (20.0%) | |
| 3 | 17 (13.9%) | 5 (18.5%) | 12 (12.6%) | |
| 4 | 2 (1.6%) | 1 (3.7%) | 1 (1.1%) | |
| ypN (n, %) | 0.603 | |||
| 0 | 62 (50.8%) | 16 (59.3%) | 46 (48.4%) | |
| 1 | 12 (9.8%) | 2 (7.4%) | 10 (10.5%) | |
| 2 | 48 (39.3%) | 9 (33.3%) | 39 (41.1%) | |
| Postoperative complications (n, %) | 12 (9.8%) | 6 (22.2%) | 6 (6.3%) | 0.024 |
| Atrial fibrillation | 2 (1.6%) | 2 (7.4%) | 0 (0.0%) | 0.048 |
| Neurological | 3 (2.5%) | 2 (7.4%) | 1 (1.1%) | 0.123 |
| Acute kidney injury | 5 (4.1%) | 3 (11.1%) | 2 (2.1%) | 0.071 |
| Vocal cord palsy | 3 (2.5%) | 0 (0.0%) | 3 (3.2%) | 1.000 |
| Hospital stay (days, IQR) | 7.0 (5.0;10.0) | 17.0 (9.0;30.5) | 6.0 (5.0;8.0) | <0.001 |
| Redamission within 30 days (n, %) | 5 (4.1%) | 5 (18.5%) | 0 (0.0%) | <0.001 |
| In‐hospital mortality (n, %) | 9 (7.4%) | 8 (29.6%) | 1 (1.1%) | <0.001 |
Note: Data are presented as mean ± standard deviation or number (%).
Abbreviations: IQR, interquartile range; SD, standard deviation; VATS, video‐assisted thoracic surgery.
A higher proportion of the patients with PPCs developed non‐pulmonary postoperative complications (22.2% vs. 6.3%, p = 0.024). The statistically significant difference was noticed with the prevalence of postoperative atrial fibrillation (7.4% vs. 0.0%, p = 0.048). Patients with PPCs had significantly longer hospital stays (with PPCs, 17 [IQR 5–9] vs. without PPCs, 7 [IQR 9.5–30.5] days, p < 0.001).
The operative mortality of the patients with PPCs was 14.8%, and all of them died because of PPCs. Patients with PPCs demonstrated significantly higher in‐hospital mortality (29.6% vs. 1.1%; p < 0.001). Readmission rate was 4.1% and all patients were readmitted because of PPCs. Among them, two patients failed to recover from PPCs; the first one was readmitted due to bronchopleural fistula and died on postoperative day 54 with massive hemoptysis. The second patient was readmitted due to the development of acute respiratory distress syndrome and died from pneumonia on postoperative day 66. There were no significant differences in other treatment characteristics and postoperative outcomes according to PPCs.
PPCs are detailed in Table 3. Twenty‐seven patients showed 43 PPCs. Among them, the most common complication was pneumonia (n = 17), followed by ARDS (n = 12).
TABLE 3.
Incidence of postoperative pulmonary complications
| Grade 2 | Grade 3a | Grade 4a | Grade 5 | Total | |
|---|---|---|---|---|---|
| Pneumonia | 3 | 1 | 3 | 10 | 17 |
| ARDS | 1 | 1 | 1 | 9 | 12 |
| BPF | 0 | 1 | 2 | 3 | 6 |
| Atelectasis | 0 | 4 | 0 | 0 | 4 |
| Prolonged air leak (>10 days) | 2 | 0 | 0 | 0 | 2 |
| Empyema | 0 | 0 | 0 | 1 | 1 |
| Pneumothorax | 0 | 1 | 0 | 0 | 1 |
| Pleural effusion | 0 | 1 | 0 | 0 | 1 |
Abbreviations: ARDS, acute respiratory distress syndrome; BPF, bronchopleural fistula.
Risk factor analysis for PPCs and construction and validation of the nomogram
Variables showing a significant difference in univariable analysis were included in the multivariable analysis (Table 4). Lower BMI (odds ratio [OR] 0.796, 95% confidence interval [CI]: 0.628–0.987, p = 0.038), no history of comorbidity (OR 0.220, 95% CI: 0.059–0.819, p = 0.048), smoking history (OR 4.362, 95% CI: 1.210–15.720, p = 0.024), and %predicted DLCO <60% (OR 3.727, 95% CI: 1.319–10.530, p = 0.013) were independently associated with PPCs.
TABLE 4.
Univariable and multivariable analyses for risk of PPCs
| Variables | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age (years) | 1.034 (0.986–1.084) | 0.171 | ||
| Male | 0.327 (0.104–1.024) | 0.055 | ||
| BMI (kg/m2) | 0.749 (0.629–0891) | 0.001 | 0.796 (0.628–0.987) | 0.038 |
| Pulmonary TBc | 3.371 (0.942–12.068) | 0.064 | ||
| No comorbidity | 0.410 (0.170–0.989) | 0.047 | 0.220 (0.059–0.819) | 0.048 |
| Age‐adjusted CCI | 1.571 (1.143–2.162) | 0.005 | ||
| Smoking history | 1.881 (1.371–17.378) | 0.014 | 4.362 (1.210–15.720) | 0.024 |
| Preop %predicted FEV1 < 60 | 3.371 (0.942–12.068) | 0.062 | ||
| Preop %predicted DLCO <60 | 2.747 (1.133–6.659) | 0.025 | 3.727 (1.319–10.530) | 0.013 |
| Clinical stage N2 (to N0–1) | 1.406 (0.478–4.133) | 0.535 | ||
| Pneumonectomy (to nonpneumonectomy) | 1.801 (0.681–4.763) | 0.232 | ||
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; DLCO, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in 1s; OR, odds ratio; PPCs, postoperative pulmonary complications; TBc, tuberculosis.
Based on the independent risk factors identified in the multivariable logistic regression, a nomogram was developed to predict the risk of PPCs (Figure 1). The discriminative performance of the nomogram for PPCs calculated using the C‐index was 0.756 (95% CI: 0.621–0.831), which demonstrated that the nomogram was accurate enough to estimate the risk of PPCs (Figure 2a). The calibration plots generated by bootstrapping presented a favorable correlation between the actual probability and the nomogram‐predicted probability (Figure 2b).
FIGURE 1.
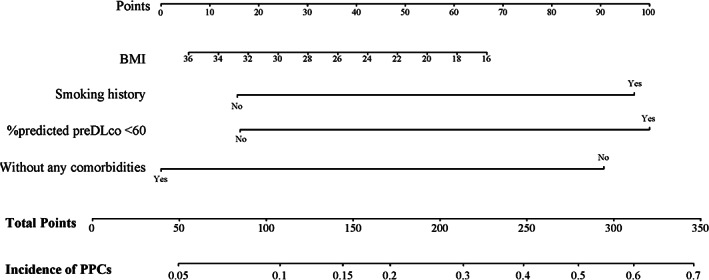
Nomogram for predicting PPCs in patients who received neoadjuvant concurrent CRTx. To use the nomogram, an individual patient's value is located on each variable axis, and a vertical line is drawn to determine the number of points received for each variable value. The sum of these points is located on the total points axis. A line is drawn downward to determine the likelihood of incidence. BMI, body mass index; CRTx, chemoradiation treatment; DLCO, diffusing capacity for CO; PPCs, postoperative pulmonary complications
FIGURE 2.
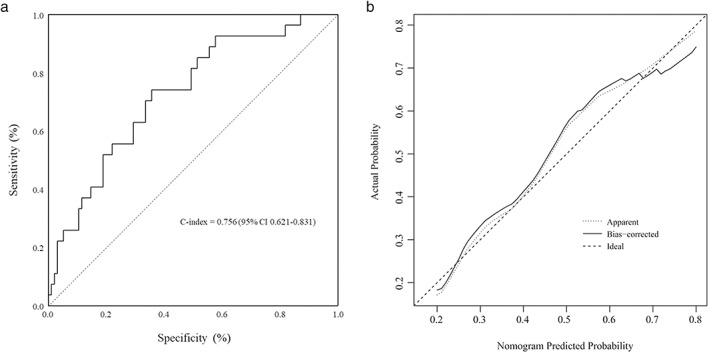
Assessment of the discriminative performance and validation of the nomogram. (a) ROC curves of the constructed nomogram. The C‐index of the model is calculated by measuring the AUC, which demonstrates that the model has a proper discriminative capacity. (b) The calibration curves generated by bootstrapping demonstrates the established nomogram have a favorable agreement with the actual probability. The dotted line (apparent) indicates the whole cohort and the solid line (bias‐corrected) is generated by bootstrapping. The ideal plot indicates perfect prediction that the nomogram predicted probability is identical to the actual probability. AUC, area under the curve; C‐index, concordance index; ROC, receiver operating characteristic
DISCUSSION
PPCs are the main cause of mortality after lung cancer surgery. The reported incidence of PPCs after lung cancer surgery varies from 15% to 37%, and PPCs are related to longer hospital stay and higher in‐hospital mortality. 9 , 10 , 21 In our study, the incidence of PPCs in patients receiving neoadjuvant CRTx was 22.1% and the in‐hospital mortality among the patients with PPCs was very high (29.6%).
Adding radiation to neoadjuvant chemotherapy is suggested to increase the perioperative morbidity and mortality rates. In the German Lung Cancer Cooperative Group study, the authors reported increased surgical mortality in patients receiving additional CRTx (neoadjuvant CTx, CRTx, surgery) than in the control group (neoadjuvant CTx, surgery) (surgical mortality, 9.2% vs. 4.5%, p = 0.11). 22 Recently, Yendamuri et al. analyzed 134 428 patients who underwent anatomical resection for NSCLC in the National Cancer Database 23 and patients who received no neoadjuvant treatment showed a lower 30‐ and 90‐day mortality rate than those who received neoadjuvant treatment (OR 0.81 and 0.74, respectively); and the patients who received CRTx showed a higher 30‐ and 90‐day mortality than those who received neoadjuvant CTx.
The changes in the pulmonary function test after neoadjuvant treatment, especially a decrease in DLCO, were significantly associated with the incidence of respiratory comorbidities. 13 , 14 Many studies have investigated the risk factors for PPCs after neoadjuvant treatment in NSCLC 9 , 10 , 11 , 12 ; however, only a few have investigated the risk factors for PPCs in patients receiving neoadjuvant concurrent CRTx. Risk factors could be attributed to the higher in‐hospital mortality of the patients with PPCs noted in our study; therefore, the identification of risk factors for PPCs during the planning of the initial treatment strategy was a pressing problem. Therefore, we identified four pretreatment independent risk‐related variables: smoking history, BMI, presence of comorbidities, and preoperative %predicted DLCO.
Smoking is a well‐known risk factor for primary lung cancer and an important predictor of PPC development. 24 The incidence of PPC is significantly higher in patients with more than 20 pack‐years of smoking history and with shorter operative smoking cessation. 23 , 24 However, recent reports have demonstrated that shorter durations of smoking cessation do not increase the risk of PPCs. 25 In our study, smoking history was found to be an independent risk factor for PPCs (OR 4.362, 95% CI: 1.210–15.720, p = 0.024).
In addition, a lower BMI has been reported as a risk factor for operative mortality and pulmonary complications after lung cancer surgery. 26 In this study, a lower BMI increased the risk for PPCs. According to the World Health Organization guidelines for the Asia‐Pacific region, we applied the cutoff value for BMI was ≤23.01 kg/m2 for differentiating normal from overweight. The overweight or obese patients have a lower risk of PPCs than normal‐weight and underweight patients do, and it might reflect the nutritional status. Various studies have found that a lower BMI was associated with impaired nutritional status, and the patients subsequently developed PPCs. 26 , 27 Impaired nutritional status is often related to respiratory muscle weakness, predisposing the patient to more PPCs. 27 Since DLCO represents the ability of alveolar gas exchange, a low %predicted DLCO may serve as an indicator of proper functioning of lung parenchyma that is important for cardiopulmonary reserve after neoadjuvant treatment and surgery.
Interestingly, pneumonectomy, one of the well‐known risk factors of PPCs,10–12 was turned out to be insignificant in this study. Patients underwent pneumonectomy reported significantly larger incidence of pneumonia when compared to nonpneumonectomy patients (21.4% vs. 4.6%, p = 0.048). However, combined incidence PPCs was not significantly different (p = 0.509), and pneumonectomy was not a significant risk factor from the univariable analysis. The total number of pneumonectomies was only 14 in the study cohort, and we assumed that this could be why pneumonectomy was eliminated during the risk factor analysis. Further study for the patients receiving neoadjuvant concurrent CRTx and pneumonectomy is needed to analyze the risk of pneumonectomy.
Our study had several limitations. First, this was a retrospective study with relatively small sample size. Second, this single‐center study was conducted at a tertiary hospital in Korea, and all patients were Asian. These factors may reduce the generalizability of the results. Third, we did not perform pulmonary function tests between neoadjuvant CRTx and surgery. Only baseline clinical data were analyzed to identify patients at risk of PPCs for deciding the overall treatment plan. Moreover, we performed lung resection approximately 7 weeks (on average) after the last induction treatment (median 7.1 weeks). Previous studies demonstrated that the onset of pulmonary symptoms from the effects of radiation is approximately 3 to 5 months after the treatment. 28 Thus, it makes sense that preoperative PFTs are more important variables to assess the risk of PPCs after neoadjuvant treatment. Finally, we performed only bootstrap validation of our model. Despite the validity of this method, a true validation with an external cohort may have better statistical power for verification. Despite these limitations, the strength of this study lies in the utilization of nomogram as a tool for selecting appropriate patients for receiving neoadjuvant therapy. It is important to identify risk factors for PPCs before treatment to ensure that this treatment strategy can be avoided in at‐risk patients. The total score can be calculated using the nomogram, and the risk of PPCs can be ealily verified. Furthermore, the nomogram showed acceptable discrimination ability for PPCs (C‐index 0.756, 95% CI 0.621–0.831), and the calibration curve demonstrated good consistency with the estimated and actual probabilities. 20 Therefore, this PPC‐predicting nomogram could be widely applied to assess the risk of PPCs in patients who received neoadjuvant concurrent CRTx followed by surgery and identify patients at an increased risk of surgery. Thus, rigorous attention should be given to patients who have been ascertained to have a high risk of PPCs by nomogram.
In conclusion, pretreatment data of DLCO, smoking history, BMI, and history of comorbidity were independent factors for PPCs in patients with neoadjuvant concurrent CRTx. The nomogram built with these factors had sufficient discriminative and predictive ability to assess the risk of PPCs in these patients. Our nomogram can help identify patients that should not receive neoadjuvant CRTx to avoid serious PPCs and also to help select patients who will benefit greatly from this aggressive treatment approach.
CONFLICT OF INTEREST
The authors all declare that they have no conflicts of interest.
ACKNOWLEDGMENTS
We would like to thank Go Eun Byun for her assistance with data collection. This work was supported by a faculty research grant of Yonsei University College of Medicine (6‐2021‐0076).
Kim HE, Yu WS, Lee CY, Lee JG, Kim DJ, Park SY. Risk factors for pulmonary complications after neoadjuvant chemoradiotherapy followed by surgery for non‐small cell lung cancer. Thorac Cancer. 2022;13:361–368. 10.1111/1759-7714.14263
Meeting presentation: Presented at the Poster Session of the IASLC 20th World Conference on Lung cancer (WCLC 2019) Barcelona, Spain, September 7–10, 2019.
REFERENCES
- 1. Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, et al. Treatment of stage III non‐small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2013;143:e314S–40S. 10.1378/chest.12-2360 [DOI] [PubMed] [Google Scholar]
- 2. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, et al. Non‐small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:504–35. 10.6004/jnccn.2017.0050 [DOI] [PubMed] [Google Scholar]
- 3. Anjali L, Christian SD, Sudesh S, Nickolas S, Jonathan S, Steven JD. Population density and facility type influence management of prostate cancer. Canc Therapy Oncol Int J. 2021;17:555973. 10.19080/CTOIJ.2021.17.555973 [DOI] [Google Scholar]
- 4. Tong S, Qin Z, Wan M, Zhang L, Cui Y, Yao Y. Induction chemoradiotherapy versus induction chemotherapy for potentially resectable stage IIIA (N2) non‐small cell lung cancer: a systematic review and meta‐analysis. J Thorac Dis. 2018;10:2428–36. 10.21037/jtd.2018.04.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Couñago F, de Dios NR, Montemuiño S, Jové‐Teixidó J, Martin M, Calvo‐Crespo P, et al. Neoadjuvant treatment followed by surgery versus definitive chemoradiation in stage IIIA‐N2 non‐small‐cell lung cancer: a multi‐institutional study by the oncologic group for the study of lung cancer (Spanish radiation oncology society). Lung Cancer. 2018;118:119–27. 10.1016/j.lungcan.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 6. Cerfolio RJ, Maniscalco L, Bryant AS. The treatment of patients with stage IIIA non‐small cell lung cancer from N2 disease: who returns to the surgical arena and who survives. Ann Thorac Surg. 2008;86:912–20. 10.1016/j.athoracsur.2008.04.073 [DOI] [PubMed] [Google Scholar]
- 7. Kim HK, Cho JH, Choi YS, Zo JI, Shim YM, Park K, et al. Outcomes of neoadjuvant concurrent chemoradiotherapy followed by surgery for non‐small‐cell lung cancer with N2 disease. Lung Cancer. 2016;96:56–62. 10.1016/j.lungcan.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 8. Stefani A, Alifano M, Bobbio A, Grigoroiu M, Jouni R, Magdeleinat P, et al. Which patients should be operated on after induction chemotherapy for N2 non–small cell lung cancer? Analysis of a 7‐year experience in 175 patients. J Thorac Cardiovasc Surg. 2010;140:356–63. 10.1016/j.jtcvs.2010.02.018 [DOI] [PubMed] [Google Scholar]
- 9. Agostini P, Cieslik H, Rathinam S, Bishay E, Kalkat MS, Rajesh PB, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax. 2010;65:815–8. 10.1136/thx.2009.123083 [DOI] [PubMed] [Google Scholar]
- 10. Yao L, Luo J, Liu L, Wu Q, Zhou R, Li L, et al. Risk factors for postoperative pneumonia and prognosis in lung cancer patients after surgery: a retrospective study. Medicine. 2021;100:e25295. 10.1097/MD.0000000000025295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roberts JR, Eustis C, Devore R, Carbone D, Choy H, Johnson D. Induction chemotherapy increases perioperative complications in patients undergoing resection for non–small cell lung cancer. Ann Thorac Surg. 2001;7:885–8. 10.1016/s0003-4975(01)02836-3 [DOI] [PubMed] [Google Scholar]
- 12. Venuta F, Anile M, Diso D, Ibrahim M, de Giacomo T, Rolla M, et al. Operative complications and early mortality after induction therapy for lung cancer. Eur J Cardiothorac Surg. 2004;31:714–7. 10.1016/j.ejcts.2007.01.017 [DOI] [PubMed] [Google Scholar]
- 13. Cerfolio RJ, Talati A, Bryant AS. Changes in pulmonary function tests after neoadjuvant therapy predict postoperative complications. Ann Thorac Surg. 2009;88:930–6. 10.1016/j.athoracsur.2009.06.013 [DOI] [PubMed] [Google Scholar]
- 14. Shin S, Choi YS, Jung JJ, Im Y, Shin SH, Kang D, et al. Impact of diffusing lung capacity before and after neoadjuvant concurrent chemoradiation on postoperative pulmonary complications among patients with stage IIIA/N2 non‐small‐cell lung cancer. Respir Res. 2020;21:1–9. 10.1186/s12931-019-1254-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization. Regional Office for the Western P . The Asia‐Pacific Perspective: Redefining Obesity and Its Treatment. Sydney: Health Communications Australia; 2020. https://apps.who.int/iris/handle/10665/206936 [Google Scholar]
- 17. Goldstraw P, Chansky K, Crowley J. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 18. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernandez FG, Falcoz PE, Kozower BD, Salati M, Wright CD, Brunelli A. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg. 2015;99:368–76. 10.1016/j.athoracsur.2014.05.104 [DOI] [PubMed] [Google Scholar]
- 20. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–70. 10.1200/JCO.2007.12.9791 [DOI] [PubMed] [Google Scholar]
- 21. Steéphan F, Boucheseiche S, Hollande J, Flahault A, Cheffi A, Bazelly B. Pulmonary complications following lung resection: a comprehensive analysis of incidence and possible risk factors. Chest. 2000;118:1263–70. 10.1378/chest.118.5.1263 [DOI] [PubMed] [Google Scholar]
- 22. Thomas M, Rübe C, Hoffknecht P, Macha HN, Freitag L, Linder A, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non‐small‐cell lung cancer. Lancet Oncol. 2008;9:636–48. 10.1016/S1470-2045(08)70156-6 [DOI] [PubMed] [Google Scholar]
- 23. Yendamuri S, Groman A, Miller A, Demmy T, Hennon M, Dexter E, et al. Risk and benefit of neoadjuvant therapy among patients undergoing resection for non‐small‐cell lung cancer. Eur J Cardiothorac Surg. 2018;53:656–63. 10.1093/ejcts/ezx406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grønkjær M, Eliasen M, Skov‐Ettrup LS, Tolstrup JS, Christiansen AH, Mikkelsen SS, et al. Preoperative smoking status and postoperative complications: a systematic review and meta‐analysis. Ann Surg. 2014;259:52–71. 10.1016/S1470-2045(08)70156-6 [DOI] [PubMed] [Google Scholar]
- 25. Lakshminarasimhachar A, Smetana GW. Preoperative evaluation: estimation of pulmonary risk. Anesthesiol Clin. 2016;34:71–88. 10.1016/j.anclin.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 26. Matsunaga T, Suzuki K, Imashimizu K, Banno T, Takamochi K, Oh S. Body mass index as a prognostic factor in resected lung cancer: obesity or underweight, which is the risk factor? Thorac Cardiovasc Surg. 2015;63:551–7. 10.1055/s-0035-1554964 [DOI] [PubMed] [Google Scholar]
- 27. Jagoe RT, Goodship TH, Gibson GJ. The influence of nutritional status on complications after operations for lung cancer. Ann Thorac Surg. 2001;71:936–43. 10.1016/s0003-4975(00)02006-3 [DOI] [PubMed] [Google Scholar]
- 28. Marks LB, Yu X, Vujaskovic Z, Small W Jr, Folz R, Anscher MS. Radiation‐induced lung injury. Semin Radiat Oncol. 2003;13:333–45. 10.1016/S1053-4296(03)00034-1 [DOI] [PubMed] [Google Scholar]


