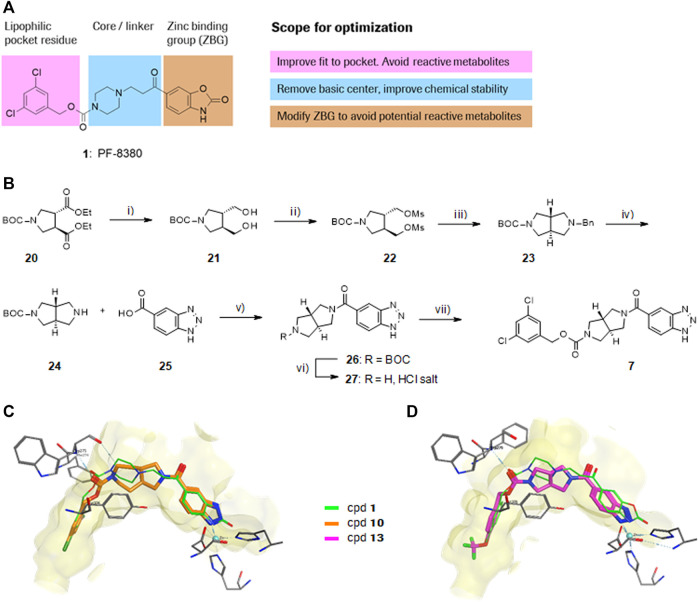
FIGURE 1.
Structure, synthesis and co-crystal structures of ATX-inhibitors. (A) Structure of PF-8380 (1) with an outline of the general approach for modification and optimization. (B) Synthesis of ATX-inhibitor 7. i) 4M LiBH4, THF, RT, 91–95% crude; ii) MsCl, NEt3, DCM, -10°C, 94% crude; iii) BnNH2, K2CO3, CH3CN, 95°C, 24 h, 53%; iv) H2, Pd/C, 1 bar, MeOH, 96%; v) HATU, NMM, DMF, RT, 16 h, 83%; vi) HCl/iPrOH (∼5–6M), RT, 87%; vii) (3,5-dichlorophenyl)methanol, CDI, NEt3, CH3CN, reflux, 87%. (C) X-ray crystal structures of human ATX in complex with 1 and 10 (overlay). Both inhibitors interact with the zinc ion in the active site (light blue) and stretch into a lipophilic pocket which corresponds to the fatty acid binding site of LPC (left). Besides the fit to the lipophilic pocket, other key interactions for 10 are the binding of the benzotriazole to the active site zinc ion and an H-bond of the carbamate oxygen to the backbone NH of Trp275. (D) X-ray crystal structures of 1 and 13 (overlay), with an essentially identical pattern of contacts with the enzyme.