FIGURE 1.
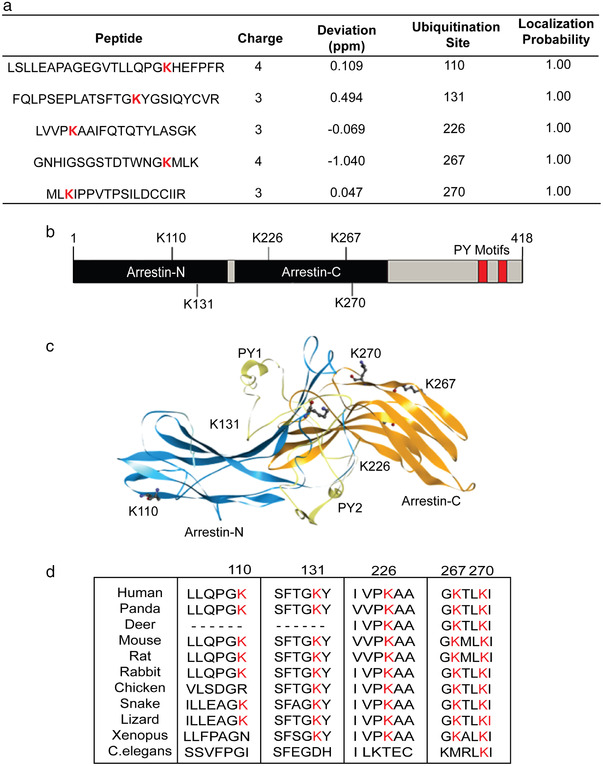
Identification of potential ubiquitinated lysine residues in Arrdc4. (a) Mass spectrometric analysis identifies five potential ubiquitination sites. (b) Schematic representation of Arrdc4 protein showing position of lysines within the Arrestin N and C domains and the relative positions of the PY motifs. (c) The structure of Arrdc4 predicted using the I‐TASSER online tool. Highlighted are the ubiquitinated K residues K110, K131, K226, K267, and K270, along with the PY motifs necessary for binding to Nedd4 family of ubiquitin ligases. (d) Multiple sequence alignment of putative Arrdc4‐like proteins from various species, showing high evolutionary conservation of K270
