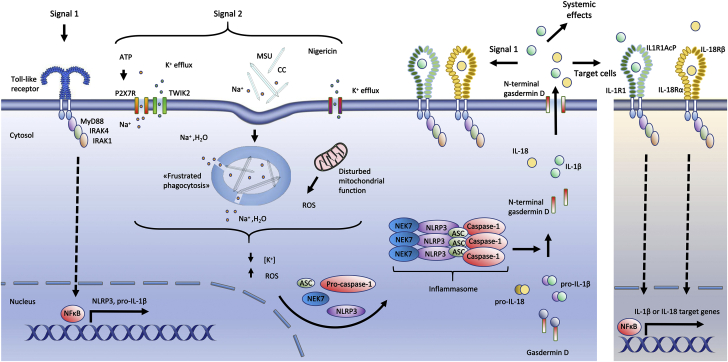
Figure 3.
Nlrp3 Inflammasome Activation
A priming signal (signal 1) indicative of the presence of microbes or tissue damage activates the transcription factor NF-κB, which induces the transcription of NLRP3 and pro-IL-1β. NLRP3 can then be activated by a wide range of molecules reflecting cellular stress (signal 2), resulting in assembly of the inflammasome, activation of caspase-1, and subsequent activation of IL-1β and IL-18. Caspase-1 also cleaves gasdermin D. The N-terminal gasdermin D peptides form a pore in the plasma membrane through which IL-1β and IL-18 are released. In more detail, extracellular ATP activates the ion channel P2X7R. This activation induces Ca2+ and Na+ influx and leads to efflux of K+ through TWIK2 (17). Monosodium urate and cholesterol crystals could activate NLRP3 via “frustrated phagocytosis,” where cells fail to eliminate engulfed crystals, resulting in lysosomal damage and leakage into the cytosol. Lysosomal Na+ and water then reduce the intracellular K+ concentration, which increase ROS production (18). In the extracellular space (right), IL-1β binds to IL-1 receptor 1 (IL-1R1), promoting heterodimerization with IL-1R accessory protein (IL-1RAcP). IL-18 binds to its receptor consisting of IL-18 receptor α (IL-18Rα) and IL-18 receptor β (IL-18Rβ). IL-1R/IL-18R may re-engage signal 1, stimulate target cells, and initiate systemic inflammatory functions such as neutrophil recruitment, production of acute-phase proteins, and fever. ATP = adenosine triphosphate; CC = cholesterol crystals; MSU = monosodium urate; NF-κB = nuclear factor κB; ROS = reactive oxygen species; other abbreviations as in Figure 2.