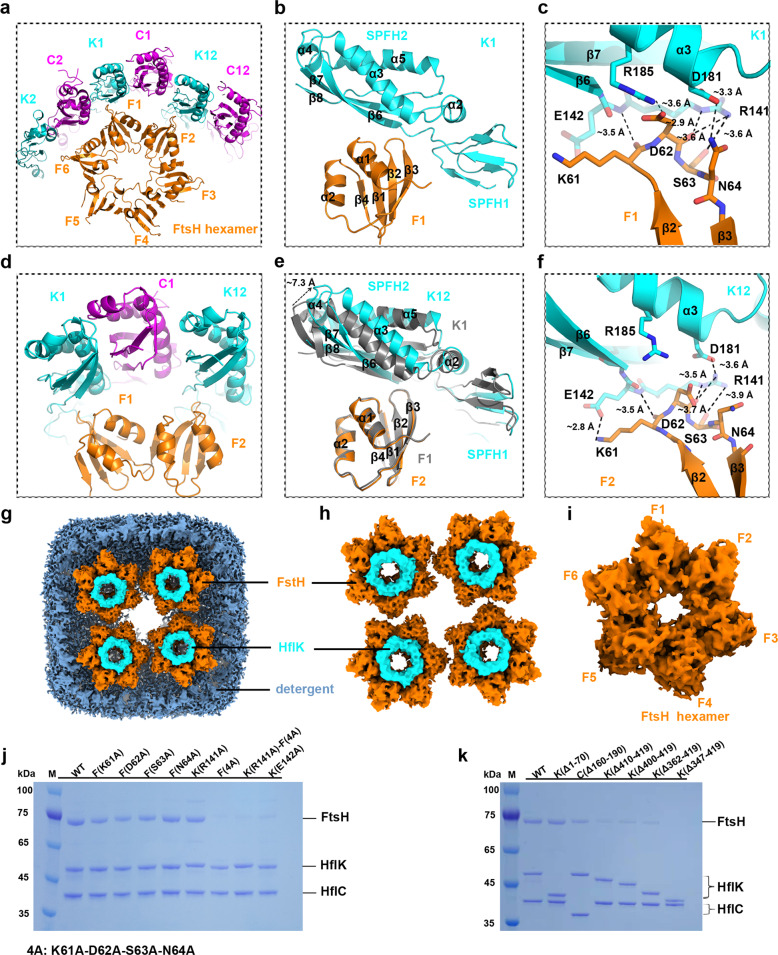
Fig. 6. Interactions between HflK and FtsH.
a Interaction between an FtsH hexamer and the inner wall of the HflK/C cage within a quarter of the KCF complex. Three FtsH periplasmic domains (F1, F2 and F6) are similarly orientated towards three copies of HflK (K1, K12 and K2), and two of them establish specific interactions. b, c Zoomed-in view showing the interactions between F1 and K1. The β2–β3 loop of FtsH contacts the linker sequence between the SPFH1 and SPFH2 domains of HflK (b). Detailed atomic interactions are highlighted in stick representation with distances labeled (c). d Same as a, with a further zoom in the two interacting FtsH periplasmic domains. e Structural comparison of the two FtsH–HflK interfaces (F1–K1 and F2–K12). Structural superimposition of F1–K1 and F2–K12 is derived using FtsH periplasmic domains as reference for alignment. F1 and K1 are colored gray. F2 and K12 were colored orange and cyan, respectively. f Same as c, but for the interface between F2 and K12. g Top view of the cryo-EM map of the KCF complex, highlighting the unmodelled density (cyan) above the hexameric periplasmic domain of FtsH. h Same as g, with detergent density removed. i Segmented cryo-EM map of a hexameric periplasmic domain of FtsH. j Point mutations on FtsH or HflK impair the binding of FtsH to the HflK/C cage. For each pull-down experiment with mutant variants, the C-terminally tagged HflC was used as bait and the loading was normalized using concentrations of HflC. k Truncations of HflK C-terminal sequences decrease the association of FtsH. For each pull-down experiment with mutant variants, the C-terminally tagged HflC was used as bait and the loading was normalized using concentrations of HflC.