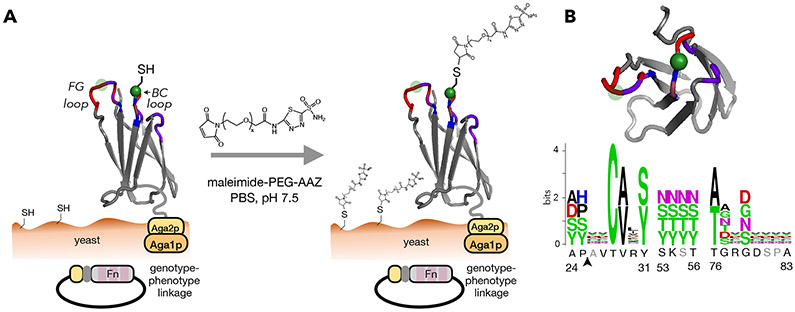
Figure 1. PriSM design integrates a pharmacophore with combinatorial protein diversity.
(A) The Fn domain is genetically diversified at up to 19 sites in three solvent-exposed loops surrounding a conserved cysteine (green). Cys28 is shown explicitly with thiol; the semi-transparent green circle indicates site 80, which was alternatively conserved in a second library. Fn is tethered to the yeast cell wall via protein fusion to a semi-flexible peptide linker to Aga2p mating protein, which is bound to Aga1p anchored in the cell wall. Treatment with maleimide-PEG-AAZ yields the protein-small molecule conjugate PriSM (as well as conjugation to other available thiols). (B) Left: top view of Fn. Right: amino acid diversity at each site with wild-type residues indicated below (gray: deleted in shortened loop lengths; arrow: insertion in lengthened loops). The Cys28 library is shown; the Cys80 library is equivalent except the diversities at sites 28 and 80 are swapped.