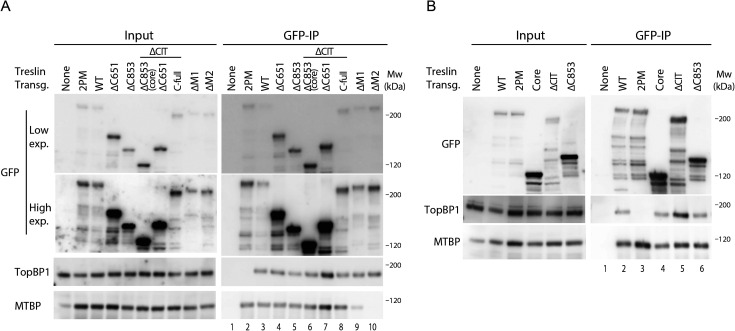
Figure S13. Treslin/TICRR-core is proficient in binding MTBP and TopBP1.
(A) The indicated GFP-Flag-Treslin/TICRR mutants were transiently transfected into 293T cells together with MTBP. Native lysates were used for anti-GFP nanobody immunoprecipitation (IP) in the presence of recombinant Cdk2-cyclin A to promote interaction with TopBP1. Lysates and bead-bound material were analysed by immunoblotting using mouse anti-GFP, rabbit anti-TopBP1, and rat anti-MTBP antibodies. Controls for IP specificity were made: Treslin/TICRR-ΔM1 and ΔM2 show decreased (M1) or absent (M2) MTBP signals, as expected. Treslin/TICRR-2PM did not bind TopBP1, as expected because the relevant CDK sites in the TopBP1/Dpb11 interaction domain are mutated to alanine. IP capabilities using (near) full-length Treslin/TICRR versions are hard to compare by immunoblotting with those containing larger deletions because of the often weak blotting efficiency of the 210 kD full-length Treslin/TICRR. However, the smaller C-terminal truncations are better comparable. Treslin/TICRR-ΔC853 and Δ651 bound similar amounts of TopBP1 and MTBP, whether they contained conserved in Treslins or not. In some experiments, however, deletion of the conserved in Treslins seemed to have a minor effect on the amount of MTBP bound (Fig 4D). (B) Independent experimental replicate of A, containing only some key samples.