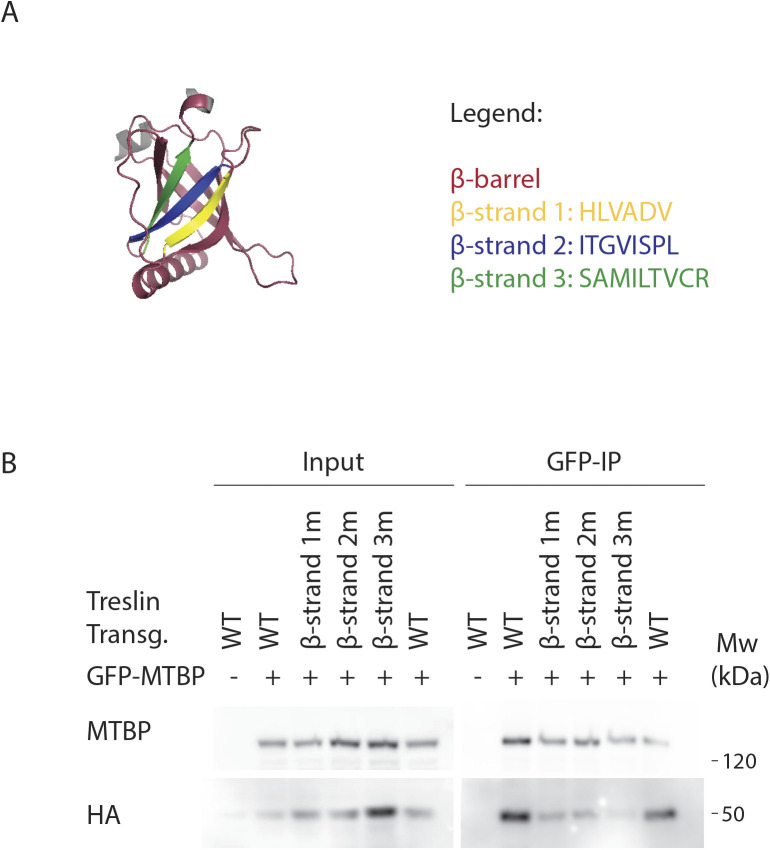
Figure S9. Mutating individual β-strands from Treslin/TICRR β-barrel compromises binding to MTBP.
(A) Treslin/TICRR Alphafold 2 model showing the β-barrel (red), including the three β-strands mutated in (B). β-strand 1 (amino acids 391–396, HLVADV, replaced with amino acids SGELRL, labeled in yellow), β-strand 2 (amino acids 405–412, ITGVISPL, replaced with amino acids SGELRLPS, labeled in blue), or β-strand 3 (amino acids 415–423, SAMILTVCR, replaced with amino acids LLCIKVEAF, labeled in green). (B) N-terminally 3HA-tagged Treslin/TICRR fragments (from amino acid 260–671) were transiently transfected into 293T together with C-terminally GFP-tagged MTBP before immunoprecipitation from cell lysates using anti-GFP nanobody immunoprecipitation. The Treslin/TICRR fragments used were WT, β-strand 1m (labeled in yellow in A), β-strand 2m (labeled in blue in A), or β-strand 3m (labeled in green in A). The Treslin/TICRR β-strand amino acids were replaced by unrelated β-strand forming sequences, to try to change the amino acid sequence without disrupting the overall structure. Results show that each β-strand mutation weakened but did not abrogate binding to MTBP, indicating that each individual β-strand may contribute to the MTBP interaction surface.