Figure 5.
Successful integration of PD-1-targeting CRISPR-Cas9 into a clinical-scale TIL-based ACT workflow
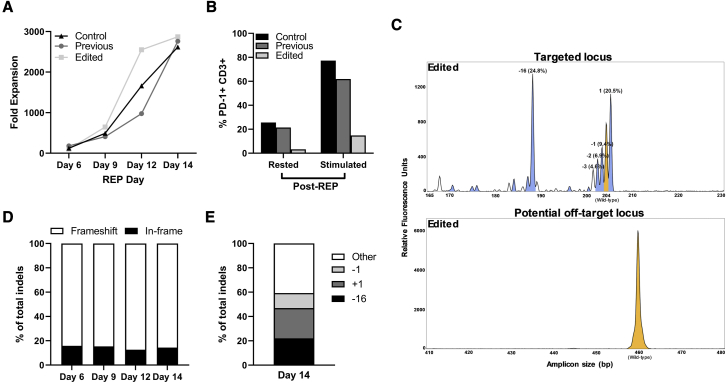
(A) Fold expansions throughout a PD-1 CRISPR-Cas9-treated (edited) clinical-scale REP compared with a previous unedited clinical-scale REP and control laboratory-scale samples. Edited sample is shown in light gray squares, previous sample in dark gray circles, and the control in black triangles. (B) Percentage of PD-1+ CD3+ cells measured post-REP via flow cytometry in samples that were either directly stained or stimulated for 48 h with aCD3/aCD28 beads. Coloring as in (A). (C) REP day 14 IDAA on-target (top) or off-target (bottom) plots for the edited sample. Yellow peaks denote unedited wild-type amplicons, blue peaks indicate frameshift indels, and white peaks indicate in-frame indels. (D) Fraction of frameshift (white bars) and in-frame indels (black) as a percentage of total indthree indels (−16 bp, black; +1 bp, dark gray; −1 bp, light gray) to total indels is shown as percentage of total indels. Remaining indels are represented by “Other” (white).