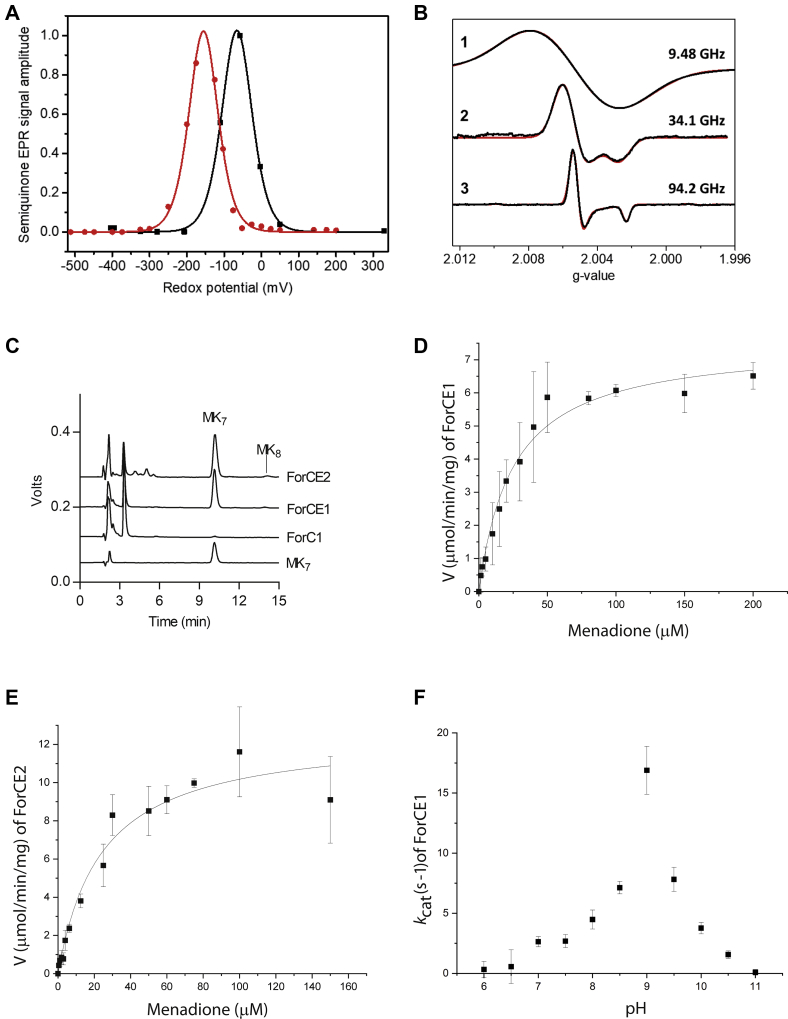
Figure 4.
ForCE1 and ForCE2 bind MK-7 as an electron sink.A, normalized redox titration curves of the MSK radical in ForCE1 buffered at pH = 6 (black) or 7.5 (red). Experimental points (filled squares or filled circles) have been fitted to the theoretical curve as described in the Experimental procedures section. Experimental conditions were as follows: temperature, 50 K, microwave power, 10 mW, and modulation amplitude, 0.5 mT (pH 7.5) or 1 mT (pH 6) at 100 kHz. B, g-scale representation of the cw EPR spectra (black traces) and their simulation (red traces) of the MSK. radical in ForCE1 buffered at pH = 6 and measured at X band (9.4812 GHz) (1), Q band (34.094 GHz) (2), and W band (94.244 GHz) (3). Spectra were simulated using the same rhombic g-tensor with principal values g1,2,3 = 2.0054, 2.0051, and 2.0023 and the linewidths given in supporting information (Table S8). Experimental conditions were as follows: temperature, 50 K, microwave power, 4 mW (1), 0.1 mW (2), or 0.05 mW (3), and field modulation amplitude at 100 kHz, 0.4 mT (1 and 3) or 0.5 mT (2). C, ForCE1 and ForCE2, but not ForC1, bind large quantities of MK-7. HPLC-ECD analysis of the MK-7 standard (15 pmol) and of lipid extracts corresponding to ∼40 pmol of purified proteins. D, Michaelis–Menten plot for the formate:menadione oxidoreduction reaction of ForCE1 at pH 9. E, Michaelis–Menten plot for the formate:menadione oxidoreduction reaction of ForCE2 at pH 9. F, pH dependence of kcat (s−1) for ForCE1.