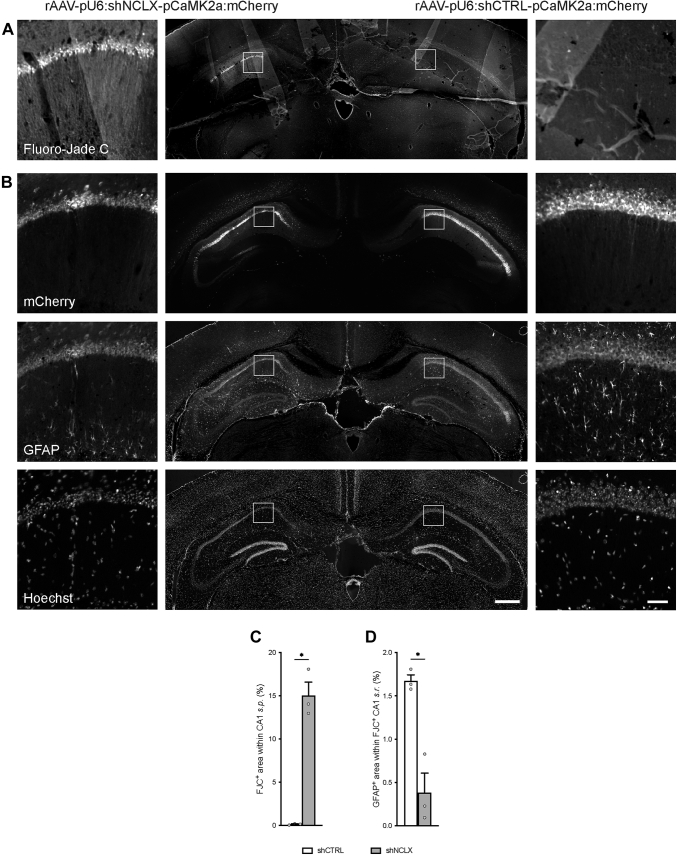
Figure 8.
shRNA-mediated NCLX knockdown leads to loss of neurons and astrocytes in vivo. C57BL/6NCrl mice were stereotactically injected with rAAVs driving the expression of shNCLX in one hemisphere and shCTRL in the other hemisphere (n = 3 mice). A, merged image of a representative tissue slice processed using FJC to stain dead and dying neurons. B, merged image of the adjacent tissue slice showing mCherry fluorescence, anti-GFAP immunochemistry, and nuclear Hoechst stain. C, quantification of the FJC+ area within infected regions of dorsal CA1 s.p. (two-tailed paired-samples t test; t(2) = 9.826, p = 0.0102). D, quantification of the GFAP+ area within FJC-labeled regions of CA1 s.r. and their counterparts within the contralateral rAAV-shCTRL-infected hemisphere (two-tailed paired-samples t test; t(2) = 4.535, p = 0.0453). The scale bars represent 1 mm (central images) and 50 μm (insets left and right). ∗p < 0.05. Bar graphs show the mean + SEM. CA1, cornu ammonis 1; FJC, Fluoro-Jade C; GFAP, glial fibrillary acidic protein; NCLX, solute carrier family 8 sodium/calcium/lithium exchanger, member B1; pCaMK2a, calcium/calmodulin-dependent protein kinase II alpha promoter; pU6, U6 small nuclear RNA promoter; rAAV, recombinant adeno-associated viral vector; s.p., stratum pyramidale; s.r., stratum radiatum.