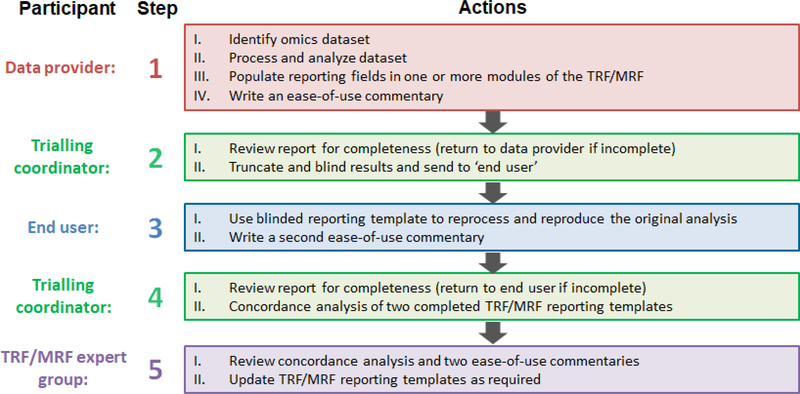
Figure 2. Approach to trialling the reporting framework.
The modules within the framework are currently being reviewed for clarity, completeness, utility and ease of use through six trials. Each trial comprises five phases, involving a data provider (initial analysis –red box), end user (re-analysis – blue box), a trialling coordinator (green boxes) and the TRF or MRF expert groups (purple box). For a module to be approved, it must enable an end user to reproduce the analysis of an omics dataset, relative to the initial analysis by the data provider.