Abstract
Study Objective:
Based on updated guidelines and expressed patient needs, we created a multidisciplinary clinic including endocrinology, gynecology/adolescent medicine, dermatology, psychology, and nutrition to provide comprehensive care to adolescent girls with polycystic ovary syndrome (PCOS). We describe the patient population presenting to this clinic and prescribing patterns when a multidisciplinary approach is utilized.
Design:
Retrospective chart review
Setting:
Tertiary care hospital
Participants:
Female patients, ages 11–24 years, presenting for initial assessment in a multidisciplinary PCOS clinic
Interventions:
None
Main Outcome Measures:
Medical history, physical exam findings, laboratory measurements and prescribed therapies
Results:
92 patients seen from 2014–2018 are described (age 15.9 years, range 11–24, BMI 35.6 kg/m2, range 19.9–53.5). Metabolic syndrome features were common: 26% had a prediabetes hemoglobin A1c (>5.6%), 83% had a high-density lipoprotein (HDL) <50 mg/dL, 40% had a systolic blood pressure >120 mmHg, and 43% had an alanine aminotransferase >30 U/L. Dermatologic findings included: acne 93%, hirsutism 38%, acanthosis nigricans 85%, hidradenitis suppurativa 16%, and androgenic alopecia 2%. 33% had a diagnosis of depression or anxiety, 16% of patients had a diagnosis of obstructive sleep apnea, and an additional 59% had symptoms warranting a sleep study The most commonly prescribed medications were topical acne preparations (62%), followed by estrogen-containing hormonal therapy (56%) and metformin (40%).
Conclusion:
In adolescents with PCOS and obesity, metabolic, dermatologic and psychologic co-morbidities are common. The use of a multidisciplinary clinic model including dermatology in addition to endocrinology, gynecology, psychology, and lifestyle experts provides care for most aspects of PCOS.
Keywords: Multidisciplinary clinic, Polycystic Ovary Syndrome, Adolescent, Dermatology, mental health, sleep health, metabolic syndrome
Introduction
Polycystic ovary syndrome (PCOS) is an endocrine disorder among women of reproductive age characterized by hyperandrogenism and chronic anovulation. PCOS is common, with an estimated prevalence of 5% to 15%.1 PCOS includes a range of clinical presentations, with variable degrees of severity and physical findings. Common clinical findings include irregular menstrual cycles, dermatologic complaints such as hirsutism, acne, acanthosis nigricans, hidradenitis suppurativa, obesity and obstructive sleep apnea, and depressed and anxious moods. Metabolic complications include insulin resistance, dysglycemia, type 2 diabetes (T2D), dyslipidemia, non-alcoholic fatty liver disease (NAFLD) and hypertension.
PCOS affects multiple organ systems, and recent studies have reinforced the need for multidisciplinary care for these patients. Metabolic abnormalities are common in adolescents with PCOS.2 The prevalence of dysglycemia increases with rising body mass index and we recently found that the incidence of T2D is high among girls with PCOS and obesity, particularly those with Hispanic ethnicity and an elevated alanine aminotransferase (ALT).3 Early cardiovascular abnormalities are present in a similar patient population.4,5 We have shown that hepatic steatosis is very common in teens with PCOS and obesity, and developed a tool to aid in diagnosis in clinic.6 Finally, we have shown that girls with PCOS are more likely to have alterations in their sleep patterns, and that in teens with PCOS, poor sleep does relate to metabolic disease.7–9 More recently, the increased prevalence of mental health concerns, including depression, anxiety, low self-esteem, negative body image, and psychosexual dysfunction has been noted in individuals with PCOS.10–12 Women with PCOS have nearly three times the rate of a psychological condition compared to those without PCOS (64% vs 24%).13 We and others have found high rates of depressive symptoms in youth with PCOS.11,14,15 As adolescents are at a relatively early stage in their disease development, they are prime candidates for primary prevention of these complications. Successful treatment of PCOS is personalized for each patient, and focusing on reducing symptom burden, metabolic complications and associated psychosocial stressors may also have an important impact on woman’s health-related quality of life.1
Following the initial diagnosis, there are many facets involved in the evaluation and treatment of an adolescent with PCOS, including: (1) regulating menses and addressing future concerns (i.e., infertility, endometrial cancer); (2) addressing metabolic abnormalities (i.e., insulin resistance, dysglycemia); (3) improving nutrition; (4) increasing physical activity, (5) addressing and treating skin findings (i.e., hirsutism, acne, hidradenitis suppurativa), and (6) behavioral counseling for poor self-image, depression, and anxiety, as well as behavior modification to successfully make lifestyle changes. Due to the complex nature of symptoms and conditions associated with PCOS, a multidisciplinary approach is necessary to address the various aspects of treatment and management. Advantages of multi-disciplinary clinics for complex care have been described in a variety of clinical settings and include: coordinated care between specialties, more rapid attainment of diagnosis and a comprehensive care plan for the patient, higher patient satisfaction with reduced numbers of appointments and time away from school and work and improved clinical outcomes.16–18 The American Family Children’s Hospital (ACFH) in Madison, WI first described a multidisciplinary adolescent PCOS clinic with practitioners consisting of pediatric endocrinologists, a health psychologist, a dietician, and a pediatric gynecologist.19,20 Another multidisciplinary PCOS clinic at University of California San Francisco (UCSF) was established in 2006 for adult women with PCOS to meet with various practitioners, as in the AFCH, but with the inclusion of dermatology. The inclusion of dermatology was crucial to fill a gap in the care of PCOS patients due to the association of PCOS with various dermatological conditions.21 Specialties represented in each of the clinics, including our own, are shown in Table 1.
Table 1:
Specialty providers in different models of multi-disciplinary PCOS clinics
| ACFH | UCSF | CHCO | |
|---|---|---|---|
| Endocrinology | V1 - Pediatric Endocrinologist | V1 - Reproductive Endocrinologist | V1 - Pediatric Endocrinologist |
| Gynecology | V1 | - | V1 |
| Nutrition | V1 | V2 | V1 |
| Psychology | V1 | V2 | V1 |
| Dermatology | - | V1 | V1 |
| Genetic Counselor | - | V1 | - |
| Exercise Specialist | - | - | V2 |
ACFH = American Family Children’s Hospital adolescent PCOS clinic, UCSF = University of California San Francisco adult PCOS clinic, CHCO= Children’s Hospital Colorado. V1= initial patient visit, V2 = follow-up patient visit.
Based on the success of the multidisciplinary approach in the treatment and management of PCOS at the AFCH and UCSF, as well as application of Endocrine Society and more recent comprehensive international PCOS guidelines,1 a novel structure for a multidisciplinary clinic for PCOS was created at Children’s Hospital Colorado (CHCO), including a team of Pediatric Endocrinologists, Gynecologists/Adolescent Medicine Specialists, Dermatologists, Psychologists, Nutritionists, and Exercise Physiologists. The role of each specialty represented in a PCOS multidisciplinary clinic is shown in Table 2. Here, we describe the structure of our multidisciplinary clinical program, patient population and lessons learned from our clinical model. The purpose of this description is to illustrate the need for such a clinic, provide details needed to create similar clinics at other sites and describe the type of medical treatment prescribed within one visit when a multi-disciplinary approach is utilized.
Table 2:
Role of providers in the evaluation of PCOS by specialty
| Specialty | Role |
|---|---|
|
| |
| Dermatology | – Hirsutism: Measure modified Ferriman-Gallwey (mFG score) |
| – Presence or absence of acne by physical exam with score of severity accordingly | |
| – Presence or absence of acanthosis nigricans, androgenic alopecia, hidradenitis suppurativa by physical exam | |
|
| |
| Gynecology/ adolescent medicine | – Chronic anovulation: menstrual irregularities and diary |
| – Clinical and/or biochemical signs of hyperandrogenism: total/free testosterone, Dehydroepiandrosterone-Sulfate | |
| – Polycystic Ovarian Morphology: pelvic ultrasound | |
| – Endometrial hyperplasia: endometrial biopsy. Discussion regarding future infertility issues | |
|
| |
| Endocrinology | – Obesity: by body mass index measurement. |
| – Hormonal disturbances: total/free testosterone, DehydroepiandrosteroneSulfate, (prolactin,17-hydroxyprogesterone, androstenedione, thyroid stimulating hormone) | |
| – Glucose intolerance / Diabetes Mellitus: glucose tolerance test, hemoglobin A1c | |
| – Dyslipidemia: lipid panel (ideally fasting) | |
| – Fatty liver: liver function test | |
| – Hypertension: measured blood pressure | |
| – Screen overweight/obese adolescents with PCOS for symptoms of obstructive sleep apnea | |
|
| |
| Psychology | – Mental health symptoms (e.g., anxiety, depression) |
| – Appetite self-regulation | |
| – Emotional eating | |
| – Goal setting for lifestyle modification | |
| – Optimizing sleep health | |
|
| |
| Exercise | – Describe goals of exercise |
| – Set activity and exercise goals at every appointment | |
|
| |
| Nutrition | – Weight trend from baseline and follow up visits |
| – Provide education regarding healthy eating habits | |
Material and methods
Study design
A retrospective chart review was conducted of adolescents and young adults with a diagnosis of PCOS presenting for their initial visit to the multidisciplinary clinic for PCOS at CHCO between 2014 to 2018. The study was approved by the Colorado Multiple Institutional Review Board.
Setting
Clinic History:
Our multidisciplinary PCOS clinic was founded in 2012 (by MCG, PH, NWA, HS, MW) and dermatology was added in 2014 (AB) at CHCO. Patients are required to have a confirmed diagnosis of PCOS prior to their first clinic visit, or a history strongly suggestive of PCOS. The clinic is held one half-day per month, and a provider from each specialty is present (Pediatric Endocrinology, Gynecology or Adolescent Medicine, Dermatology, Psychology, Nutrition/Exercise Physiology).
From 2012–2014, we followed the initial AFCH structure, with 8 patients per clinic. Each patient saw every specialty during the visit. Specialists and topics addressed in clinic were designed to follow the most recently published Endocrine Society PCOS guidelines.22 During this time, gynecology and endocrinology specialists found that each were providing extensive basic teaching on PCOS etiology and treatment, other specialty providers found more success in engaging patients in lifestyle interventions if their interactions occurred following this basic teaching, and patients complained that the visits were too long.
When dermatology was added in 2014, we significantly restructured the clinic to address the above concerns. The clinic also physically moved into a dedicated multidisciplinary clinic space with a classroom, a large provider workroom to enable easy communication and collaboration, and procedure rooms to accommodate placement of contraceptive implants. Patients seen after these changes were made are described in this manuscript.
Our goals in moving to the multi-disciplinary clinic space were to (1) engage in group education and thereby reduce time providers each spent in individual and overlapping counseling, (2) create more time and space for providers to collaborate on patient care in the shared workroom, and (3) expand procedural needs for patients. We therefore adopted a class model with 6–7 patients and families presenting to clinic for a 90-minute group education session prior to their individual clinic visits. The first 45 minutes were taught by Endocrinology and Gynecology and reviewed the pathophysiology of PCOS and medical treatment approaches. The remaining 45 minutes were taught by Nutrition and Exercise Physiology and reviewed lifestyle recommendations.
Over the next two years, we solicited and responded to feedback from patients and providers to improve clinic efficiency. This included adding an additional psychologist and endocrinologist and dividing patients into new and follow up cohorts to avoid repeating educational content. The current model features a 75-minute group class for 6–7 new patients, who are then roomed for their individual appointments, and then 6–7 follow-up patients who have individual appointments with all specialists. The power point slides utilized for the group class and a schematic of the current clinic structure. For Spanish-speaking patients, a Spanish interpreter is present for the group class. Patients with developmental delays that would limit applicability of the curriculum are seen in clinic but do not participate in the class.
With COVID restrictions in 2020 (initiated after data collection for this manuscript), we had to stop providing the in-person class and recorded the content for patients to watch prior to their visit. We plan to continue to offer this option even after COVID restrictions are lifted.
Study Population and Clinical Measures
New patients seen in PCOS clinic after 2014 were identified. Data collected from the initial PCOS clinic visit included: age, demographic information, weight, height and body mass index (BMI), cutaneous findings, diagnosed associated comorbidities, diagnostic tests and medical treatment provided. Each chart was independently reviewed by at least two individuals for accuracy and completeness of data collection (DS, JB and MCG). Laboratory data were included if they had been collected within six months of the visit date.
Hirsutism was evaluated by Ferriman Gallwey score (FGS) or, when not documented numerically, evaluated qualitatively as none (FGS 0–5), mild (FGS 6–11), moderate (FGS 12–16), or severe (FGS >17). FGS score data were available for 79 of the 91 individuals. Acanthosis nigricans at the neck was scored on a scale of 0–4, with 0 as none, 1 barely visible at the back of the neck, 2 extending across the back of the neck, 3 as extending around the sides of the neck, and 4 circumferential. Data were present for 78 of the 91. Acne was scored by subtype (comedonal, inflammatory, mixed, or nodulocystic) and assigned a value on a scale of 0–4, with 1 being the mildest, or comedonal, and 4 being the most severe, or nodulocystic. Acne data was available for 89 of the 91 individuals. BMI was calculated as kilograms divided by height in meters squared (≥ 25 kg/m2 overweight, ≥30 kg/m2 obese). Additionally, for participants under 20 years, pediatric norms for BMI were used (percentile, where 5 to <85% is normal weight, 85 to <95% is overweight and ≥95% is obese). The 2000 CDC Growth Charts were used to calculate percentiles.23
Laboratory measures and systolic blood pressure were categorized as normal or abnormal based on laboratory assay reference ranges or guidelines. Elevated free testosterone was defined as >6.3 ng/dL (LC/MSMS + equilibrium dialysis, Esoterix division of Labcorp, San Diego, CA). Elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were defined as >36 IU/L and 30 IU/L, respectively (CHCO, clinical lab, standard methods). Dyslipidemia was defined as HDL <50 ng/dL and/or triglycerides >150 mg/dL. Elevated systolic blood pressure is defined as >120 mmHg per recent American Heart association guidlines.24 Low vitamin D is defined as <20 ng/dL per Endocrine Society Guidelines, and was checked in the setting of significant obesity, depression or a diet low in calcium containing foods.25 Pre-diabetes is defined as hemoglobin A1c (HbA1c) between 5.7–6.4% per the American Diabetes Association guidelines, and diabetes as ≥6.5%.26
Prescribing patterns were evaluated, and hormonal methods included: combined contraceptive methods, medroxyprogesterone or long-acting progestin contraception. Dermatologic treatments included topical treatments: benzoyl peroxide, salicylic acid, clindamycin, 12% Ammonium lactate cream, 4% Chlorhexidine gluconate, 5% Minoxidil, 13.9% Eflornithine HCl cream, Tretinoin; oral treatments included: spironolactone, oral antibiotics, isotretinoin.
Statistical analysis
Descriptive data are presented as number and percent. Percentages are calculated for non-missing data. Normally distributed data are presented as mean and standard deviation and nonparametric data as median an interquartile range. Data were analyzed using Graph pad Prism V8.4.3 (San Diego, CA).
Results
Demographic information, frequency of dermatological conditions, and other comorbidities are listed in Table 3. In total, data from 92 patients under the age of 18 years were included. The majority of patients were of white race (86%) and Hispanic ethnicity was common (49%). Patient ages ranged from 11 to 24 years. As the clinic is housed in the CHCO obesity program, the majority of patients were overweight or obese and BMI ranged from 19.9 to 53.5 kg/m2. Age of menarche ranged from 8 to 16 years.
Table 3:
Patient characteristics
| Measurement | N = 92 |
|---|---|
| Age (years) | 15 (14,17) |
| Race (N, % yes) White Black Asian Multiracial |
76 (87%) 8 (9%) 2 (3%) 1 (1%) |
| Ethnicity Hispanic (N, % yes) |
43 (49%) |
| Menarche (years) | 12 (11,12) |
| Metabolic | |
| BMI (kg/m2) | 35.3 (31.3, 40.0) |
| BMI Percentile* | 98.6 (97, 99) |
| Liver edge palpated beyond costal margin (N, % yes) | 16 (19%) |
| Systolic blood pressure (mmHg) | 118 (116,122) |
| Elevated Systolic blood pressure (N, %) | 36 (40) |
| Diastolic blood pressure (mmHg) | 66 (64,74) |
| Diagnosis of type 2 diabetes (N, % yes) | 6 (7%) |
| Sleep study necessary (N, % yes) | 55 (61%) |
| Sleep study ordered (N, % yes) | 20 (22%) |
| Sleep study performed (N, % yes) | 16 (18%) |
| Obstructive sleep apnea (N, % yes) | 14 (16%) |
| Mental Health | |
| Depression diagnosis (N, % yes) | 26 (28%) |
| Anxiety diagnosis (N, % yes) | 14 (15%) |
| Dermatologic | |
| Hirsutism (N, % yes) | 35 (38%) |
| Hirsutism (FGS score) | 4 (2, 9) |
| Acne (N, % yes) | 85 (91%) |
| Acne severity (N, 0–4, ND) | (5/38/24/16/5/3) |
| Acanthosis nigricans | 78 (85%) |
| Acanthosis nigricans at neck (N, 0–4, ND) | (3/11/23/21/20/14) |
| Hidradenitis suppurativa (N, % yes) | 14 (16%) |
| Androgenic pattern scalp alopecia (N, % yes) | 2 (2%) |
BMI percentile only available for girls ≤ 18 years, N= 87, FGS= Ferriman Gallwey score, ND= not documented, Elevated Systolic blood pressure > 120 mm/hg
An elevated systolic blood pressure was present in 40% of patients and six patients (7%) had type 2 diabetes. Sleep abnormalities were common, with 16% presenting with a previous diagnosis of obstructive sleep apnea, and another 22% being referred for diagnostic polysomnogram testing. Almost a third of patients had a pre-existing diagnosis of mental health disorder, including depression (28%) and anxiety (15%).
Dyslipidemia was common, characterized by a low HDL and elevated triglyceride concentrations (Table 4). In total, 89% of patients had at least one marker of metabolic syndrome (low HDL, high triglycerides, prediabetes HbA1c or high systolic blood pressure) in addition to obesity. Additionally, ALT was elevated in 66% of patients and AST in 43% of patients. Vitamin D deficiency was present in 19% of patients tested, although data were only available in 32 patients (35%).
Table 4:
Laboratory measures
| Test | Participants with data (N, % of total cohort) | Average values | % abnormal (N, % of test) |
|---|---|---|---|
| Free testosterone (pg/mL) | 84 (91%) | 7.8 (5.5, 11) | 58 (69%) |
| HDL (mg/dL) | 83 (90%) | 39 (34,46) | 69 (83%) |
| TG (mg/dL) | 84 (91%) | 138 (96, 181) | 39 (46%) |
| HbA1c (%) | 91 (99%) | 5.4 (5.2, 5.7) | 24 (26%)a |
| Vitamin 25OH-D (ng/mL) | 32 (35%) | 28.5 ± 8.3 | 6 (19%) |
| AST (IU/L) | 76 (83%) | 33 (25, 51) | 33 (43%) |
| ALT (IU/L) | 82 (89%) | 32 (29, 48) | 54 (66%) |
Laboratory data are shown as median (25,75%ile) or mean ± standard deviation of the mean. ALT= alanine aminotransferase, AST= aspartate aminotransferase, HDL= high density lipoprotein, TG= triglycerides. Cutoffs for abnormal values per local assays and/or per guidelines: free testosterone >6.3 ng/dL, HDL <50 mg/dL, TG >150 ng/dL, HbA1c >5.6%, Vitamin 25OH-D <20 ng/dL, AST >36 IU/L, ALT >30 IU/L.
2 of 6 patients with type 2 diabetes had an HbA1c >6.5%
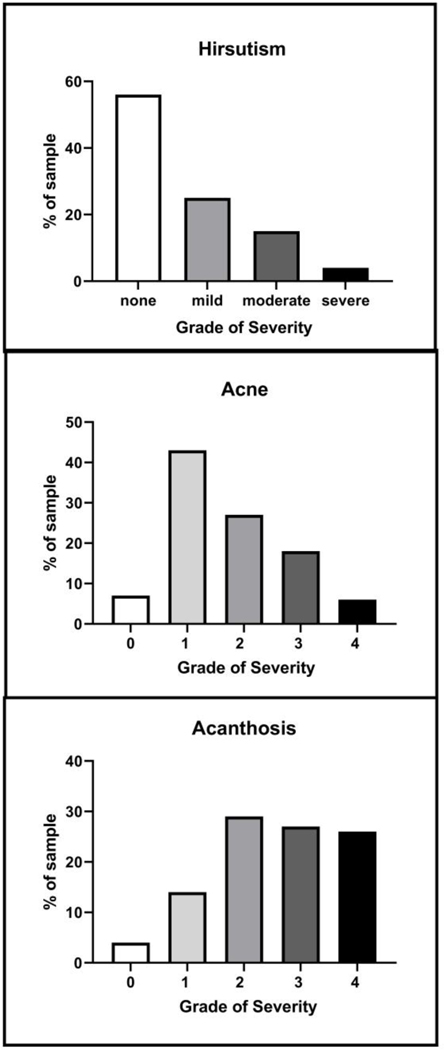
Of note, 97% (n=90) had at least one dermatological skin condition typically associated with PCOS. Especially common in the patient population were acne, hirsutism and acanthosis nigricans (Table 3). A majority of the adolescent girls in the study had acne, with over half of the reported cases being comedonal; more severe (nodulocystic or inflammatory) was the least common (Figure 1A). Thirty-eight percent of patients had documented hirsutism, with 22% mild and 16% moderate to severe (Table 3 and Figure 1B), although 12 did not have an FGS documented. Acanthosis nigricans was also prevalent (85%) and tended to be moderate to severe (Figure 1C).
Figure 1: Severity of Dermatologic Findings.
Data shown are percentage of each category of severity, from the number of patients with data. Hirsutism (N=79 by Ferriman Gallwey score (FGS) as none (FGS 0–5), mild (FGS 6–11), moderate (FGS 12–16), or severe (FGS >17); Acne (N=89), categorized into none, comedomal =1, inflammatory =2, mixed comedomal or inflammatory =3, or nodulocystic =4. Acanthosis Nigricans (N=78), with 0 corresponding to none, 1 barely visible at the back of the neck, 2 extending across the back of the neck, 3 as extending around the sides of the neck, and 4 circumferential.
A variety of medications were prescribed prior to or at the initial multidisciplinary appointment to treat various phenotypes of PCOS (Table 5). The most common medications prescribed among PCOS patients were for acne treatment. Seventy-six percent of patients received some form of dermatologic treatment, and topical acne medications were prescribed to more than half of all patients (58%), with especially high use of tretinoin (52%). Following acne treatments, several of the most common medications targeted multiple areas of improvement in PCOS. Less commonly prescribed medications for dermatologic indications: spironolactone, 13.9% eflorithine cream, laser hair removal for hirsutism, alpha-hydroxy lotions for acanthosis nigricans, and 4% chlorhexidine gluconate solution for hidradenitis suppurativa.
Table 5:
Medications prescribed prior to or at initial visit
| Medication | Number (%) |
|---|---|
| Hormonal modulator | |
| Combined oral contraceptives | 52 (57%) |
| Medroxyprogesterone challenge | 18 (20%) |
| Progestin LARC (IUD or Implant) | 6 (7%) |
| Metabolic/Other | |
| Metformin | 37 (40%) |
| Anti-Depressant/Anti-Anxiety | 12 (13%) |
| Lipid modulating medication (stain/fibrate/omega 3s) | 1 (1%) |
| Dermatologic | |
| Spironolactone | 12 (13%) |
| Topical acne medication Tretinoin |
53 (58%) 48 (52%) |
| Oral dermatologic medication Isotretinoin Oral antibiotic |
18 (20%) 4 (4%) 14 (16%) |
| 13.9% Eflornithine HCl cream | 10 (11%) |
| Laser hair reduction | 7 (8%) |
| 12% Ammonium lactate cream for acanthosis | 15 (17%) |
| 4% Chlorhexidine gluconate cleanser for hidradenitis | 11 (12%) |
| 5% Minoxidil topical solution for alopecia | 1 (1%) |
| Medication Classes and Combinations | |
| Metformin only | 3 (3%) |
| Dermatologic only | 13 (14%) |
| Hormonal only | 10 (11%) |
| Hormonal + Metformin | 4 (4%) |
| Hormonal + Dermatologic | 27 (29%) |
| Hormonal + Metformin + Dermatologic | 24 (26%) |
| Metformin + Dermatologic | 6 (7%) |
| Lifestyle only, no medications | 5 (5%) |
LARC = long-acting reversible contraceptive, IUD= intrauterine device. Hormonal methods included: combined contraceptive methods, medroxyprogesterone or long-acting progestin contraception. Dermatologic treatments included topical treatments: benzoyl peroxide, salicylic acid, clindamycin, 12% Ammonium lactate cream, 4% Chlorhexidine gluconate, 5% Minoxidil, 13.9% Eflornithine HCl cream, Tretinoin; oral treatments included: spironolactone, oral antibiotics, isotretinoin.
Over half (57%) of patients were prescribed combined oral contraceptive pills for menstrual regulation and endometrial protection, as well as treatment of acne or hirsutism in a smaller cohort of these patients. No patient received a prescription for combined hormonal contraceptive patches or vaginal rings. Twenty percent of patients were prescribed a medroxyprogesterone challenge to induce menstrual bleeding, with refills in case of continued anovulation despite lifestyle therapy for 9% and medroxyprogesterone followed by combined hormonal contraception in 11%. Long-acting progestestin-based contraceptives were used by a minority of individuals (7%). Metformin was prescribed commonly (40%) with the target dose of 2,000 mg a day.
The majority of patients received more than one prescription, and only five patients opted for lifestyle counseling alone. Twenty-four (26%) received four prescriptions, followed by 23% with three, 14% with two and 15% with only one prescription. Twenty-one percent received more than four prescriptions. Specific combinations of metformin, hormonal and dermatologic therapies were also examined, and are shown in Table 5. The most common combination was hormonal + dermatologic (29%), followed by hormonal + dermatologic + metformin (26%). Metformin and psychologic medications were exclusively prescribed by endocrinology, and hormonal therapies split between endocrinology and gynecology. Spironolactone was prescribed by endocrinology, gynecology and dermatology. Topical treatments, antibiotics and isotretinoin were exclusively prescribed by dermatology.
Discussion
Here we present an overview of our PCOS multidisciplinary clinical program, clinical and laboratory information on patients presenting for their initial appointment at the clinic and prescribed treatments within the first visit. Dermatologic manifestations were very common, with topical acne medications being the leading medication class prescribed. Consistent with previous reports, regulation of menses was a primary concern, with hormonal therapy being the most prescribed oral medication, with cyclic and long-term progestins also prescribed. We have previously shown that subdermal long acting progestin therapies are well tolerated in girls with PCOS and obesity.27 Sixty-six percent of patients received combinations of medications traditionally prescribed by more than one specialty, highlighting the importance and effectiveness of integrating dermatologist(s) and gynecologist(s)/adolescent medicine physicians in a multidisciplinary clinic to address these concerns in a timely manner. Eighty-nine percent of patients had evidence of some form of metabolic disease beyond obesity, with 40% prescribed metformin, underscoring the need for interventionists trained in lifestyle modification, including endocrinologists, psychologists, dieticians, and exercise specialists. We found that nearly a third of participants had a mental health diagnosis, emphasizing the need for a psychologist. We have previously published our analysis of a formal depression scale, which found that 60% of girls with PCOS and 78% of girls with PCOS and T2D have elevated depressive symptoms.28 It is important to note that as a referral clinic, it is likely that the patients seen in this clinic are more likely to have multiple symptoms of PCOS and be viewed as more complex than patients who are not referred and followed either solely by their primary care provider or in conjunction with Gynecology, Adolescent Medicine or Endocrinology clinics.
The multidisciplinary clinic for PCOS at AFCH is one of the oldest adolescent PCOS clinics in the country, established in 2005. The practitioners characterized 70 patients over five years20 and found hirsutism in 60% of the patients, as well as other common comorbidities such as primary and secondary amenorrhea, insulin resistance, and dyslipidemia, similar to our patient population. They also found that over 11 years, the clinic had 70% of returning patients demonstrate weight loss or stabilization, with 57% succeeding at weight loss.19 Thus, a significant difference was shown in short-term outcomes of adolescent females with PCOS with the multidisciplinary clinic as compared to general weight loss data in youth, demonstrating the clinics usefulness as a method of intervention.
The PCOS clinic at UCSF examined cutaneous features including acne, hirsutism, acanthosis nigricans and androgenic alopecia in 401 adult women referred primarily for infertility concerns over 6 years. Ninety-one percent of women meeting the PCOS criteria had at least one skin condition. Hirsutism and acanthosis nigricans were associated with both biochemical hyperandrogenism, and several metabolic abnormalities, including glucose intolerance, higher BMI, and dyslipidemia (high triglycerides and low HDL).21 Their findings are similar to ours, as we also found high percentages of hirsutism and acanthosis nigricans (Table 3) and metabolic disease, particularly higher triglycerides and low HDL (Table 4).
Both the AFCH and UCSF clinics served as a model for the development of the multidisciplinary PCOS clinic at our institution, and faculty within the clinics shared their experiences in the initial setup of our clinic. However, unlike the UCSF PCOS clinic, which has a focus on fertility of adult women with PCOS, the PCOS clinic at CHCO has a focus on lifestyle and clinical aspects of PCOS. When different types of specialists treat PCOS in isolation, prescribing practices may reflect the bias and experience of the provider, and may not be the choice the patient would pick, if presented with all options equally. For example, endocrinologists have a bias towards metformin, and gynecology and adolescent medicine towards hormonal therapies.29,30 We thus sought to develop a clinic that would bring together all specialties in a collaborative and patient-oriented setting. Our data of high rates of cross-discipline combination therapy are in contrast to that seen when adolescent patients are seen by gynecology or endocrinology in isolation.29,30 This cross-over of disciplines is critical in PCOS. For example, addressing a patient’s skin concerns can have an positive impact of adequate treatment in their overall wellbeing and self-esteem.31 Acne in adolescents has shown to intensify feelings of guilt, shame, and social isolation,32 and patients with acne had 46% higher risk for major depression compared to controls.33 Further, estrogen containing therapies can also improve dermatologic conditions such as hirsutism and cystic acne in addition to gynecologic benefits.34 Including dermatology extends PCOS treatment beyond the typically prescribed metformin and hormonal therapies. Further study is needed to determine if rapid treatment of cosmetic concerns leads to improvements in self-esteem or mood. Exercise as part of lifestyle modification is beneficial for treatment of PCOS as it aids in improving insulin sensitivity, dyslipidemia, and body composition, as well as mental health beneifts.5,6 The PCOS clinic at CHCO is, to our knowledge, the first adolescent PCOS clinic in the US to incorporate dermatology and encompass the full potential of this complimentary and comprehensive clinical model.
It is important to note that pediatric psychology in this clinic model is not present only for mood-based assessments. When pediatric psychologists are embedded within the PCOS clinic paradigm, they evaluate for psychosocial factors contributing to overweight status, assess for co-occurring psychological considerations and maximize adherence with lifestyle modifications. The role of pediatric psychology utilizes a semi-structured clinical interview format, termed the “Obesity Behavioral Risk Profile and Intervention,” that assesses behavioral factors contributing to overweight status. The assessment and intervention consultation is conducted in a family-based setting, inclusive of family members consistent with research underscoring the importance of including family members in the treatment of youth obesity.35 Thus, it is important to include practitioners who have been adequately trained in all of these assessments to fully compliment and Integrate patientcare with the rest of the PCOS team.
Multidisciplinary care with this type of model requires institutional commitments, including administrative resources, adequate space and possible revenue-based allowances for not meeting target goals. For example, in a cancer based multidisciplinary care clinic, whereas provider and patient satisfaction were improved, provider efficiency was decreased.16 In our model, the endocrinology, nutrition and psychology providers are seeing the expected number of patients per clinical session, however the number of patients for the amount of time spent in clinic is low for gynecology and dermatology providers. Both the Endocrine Society and the American College of Gynecology guidelines recommend dermatologic treatments, and the 2018 PCOS guidelines include many specific recommendations for administering lifestyle counseling and assessing mood and quality of life.1,36,37 However, implementation of this type of care will necessitate more than guidelines. Significant provider education and development of electronic medical based tools will be required, as we have demonstrated was needed for consistent PCOS diagnosis and metabolic screening across specialties.38
There are several limitations of this study. As is the case with any retrospective chart review, data were missing for some outcomes. For example, we included laboratory values that were collected within six months of the first visit. However, changes in that time period could affect lab values. Additionally, data were collected from the first visit to the PCOS clinic, but often this was not the time of diagnosis of PCOS. Many patients had seen other providers prior to being referred and started medications and treatments prior to their initial PCOS clinic visit. We did not include psychosocial measures or formal assessments of mood, as these were not implemented until 2016. However, 44 patients in this manuscript are included in another by our group showing high rates of depressive symptoms.28 As this was a cross-sectional retrospective chart review, we did not have longitudinal data on bleeding patterns or changing medication regimens. This retrospective cohort has several strengths. Consistent guidelines for diagnosis of PCOS were used in the clinic and thus all patients meet the Endocrinology Society guidelines with adolescent adaptations.22 Furthermore, our patient population is fairly diverse, with a large percentage of Hispanic patients, although we have relatively few Black patients, as reflective of the Colorado population. Finally, it is important to note that the rates of signs, symptoms and comorbidities associated with PCOS in this population are high and likely reflective of the more complex patients referred to PCOS clinic and not representative of the general population of adolescents with PCOS and obesity.
Multidisciplinary care for girls with PCOS is valuable for treating all aspects and comorbidities of PCOS, from management of menses to mental health. With the diversity of manifestations among girls with PCOS, a range of practitioners allows for all patient concerns to be addressed. Despite the presence of other adolescent multidisciplinary PCOS clinics, dermatology has not, to our knowledge, been included in multidisciplinary care. We have shared our experience and type of patients in the belief that this type of model provides the most comprehensive care and can be implemented at other sites.
Acknowledgments:
The authors would like to acknowledge the efforts of the rest of the PCOS team, in particular Angela Besko, RN, and administrators Alyssa Jumps and Lerin Payne for their assistance with this clinic.
Funding: MCG: NIDDK T32 DK063687, BIRCWH K12HD057022, K23DK107871 Doris Duke Foundation 2015212 and Children’s Hospital Colorado. NJN: NIDDK T32 DK063687, BIRCWH K12HD057022, Doris Duke Foundation 2015212. SLS: BIRCWH K12HD057022, K23DK117021.
Footnotes
Disclosures: MCG has served as a consultant for Novo Nordisk. None of the authors have anything to disclose or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akgul S, Bonny AE. Metabolic Syndrome in Adolescents with Polycystic Ovary Syndrome: Prevalence on the Basis of Different Diagnostic Criteria. Journal of pediatric and adolescent gynecology. 2019;32(4):383–387. [DOI] [PubMed] [Google Scholar]
- 3.Poomthavorn P, Chaya W, Mahachoklertwattana P, Sukprasert M, Weerakiet S. Glucose metabolism in obese and lean adolescents with polycystic ovary syndrome. Journal of pediatric endocrinology & metabolism : JPEM. 2013;26(3–4):319–324. [DOI] [PubMed] [Google Scholar]
- 4.Patel SS, Truong U, King M, et al. Obese adolescents with polycystic ovarian syndrome have elevated cardiovascular disease risk markers. Vasc Med. 2017:1358863X16682107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fruzzetti F, Ghiadoni L, Virdis A, et al. Adolescents with Classical Polycystic Ovary Syndrome Have Alterations in the Surrogate Markers of Cardiovascular Disease but Not in the Endothelial Function. The Possible Benefits of Metformin. Journal of pediatric and adolescent gynecology. 2016;29(5):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carreau AM, Pyle L, Garcia-Reyes Y, et al. Clinical prediction score of nonalcoholic fatty liver disease in adolescent girls with polycystic ovary syndrome (PCOS-HS index). Clinical endocrinology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon SL, McWhirter L, Diniz Behn C, et al. Morning Circadian Misalignment Is Associated With Insulin Resistance in Girls With Obesity and Polycystic Ovarian Syndrome. The Journal of clinical endocrinology and metabolism. 2019;104(8):3525–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon SL, Behn CD, Cree-Green M, et al. Too Late and Not Enough: School Year Sleep Duration, Timing, and Circadian Misalignment Are Associated with Reduced Insulin Sensitivity in Adolescents with Overweight/Obesity. The Journal of pediatrics. 2019;205:257–264 e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon S, Rahat H, Carreau AM, et al. Poor Sleep Is Related to Metabolic Syndrome Severity in Adolescents With PCOS and Obesity. The Journal of clinical endocrinology and metabolism. 2020;105(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffey S, Mason H. The effect of polycystic ovary syndrome on health-related quality of life. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2003;17(5):379–386. [DOI] [PubMed] [Google Scholar]
- 11.Almis H, Orhon FS, Bolu S, Almis BH. Self-Concept, Depression, and Anxiety Levels of Adolescents with Polycystic Ovary Syndrome. Journal of pediatric and adolescent gynecology. 2020. [DOI] [PubMed] [Google Scholar]
- 12.Sari SA, Celik N, Uzun Cicek A. Body Perception, Self-Esteem, and Comorbid Psychiatric Disorders in Adolescents Diagnosed with Polycystic Ovary Syndrome. Journal of pediatric and adolescent gynecology. 2020;33(6):691–696. [DOI] [PubMed] [Google Scholar]
- 13.Coffey S, Bano G, Mason HD. Health-related quality of life in women with polycystic ovary syndrome: a comparison with the general population using the Polycystic Ovary Syndrome Questionnaire (PCOSQ) and the Short Form-36 (SF-36). Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2006;22(2):80–86. [DOI] [PubMed] [Google Scholar]
- 14.Deeks AA, Gibson-Helm ME, Teede HJ. Anxiety and depression in polycystic ovary syndrome: a comprehensive investigation. Fertility and sterility. 2010;93(7):2421–2423. [DOI] [PubMed] [Google Scholar]
- 15.Coban OG, Tulaci OD, Adanir AS, Onder A. Psychiatric Disorders, Self-Esteem, and Quality of Life in Adolescents with Polycystic Ovary Syndrome. Journal of pediatric and adolescent gynecology. 2019;32(6):600–604. [DOI] [PubMed] [Google Scholar]
- 16.Bunnell CA, Weingart SN, Swanson S, Mamon HJ, Shulman LN. Models of multidisciplinary cancer care: physician and patient perceptions in a comprehensive cancer center. Journal of oncology practice / American Society of Clinical Oncology. 2010;6(6):283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grosse SD, Schechter MS, Kulkarni R, Lloyd-Puryear MA, Strickland B, Trevathan E. Models of comprehensive multidisciplinary care for individuals in the United States with genetic disorders. Pediatrics. 2009;123(1):407–412. [DOI] [PubMed] [Google Scholar]
- 18.Ajarmeh S, Er L, Brin G, Djurdjev O, Dionne JM. The effect of a multidisciplinary care clinic on the outcomes in pediatric chronic kidney disease. Pediatr Nephrol. 2012;27(10):1921–1927. [DOI] [PubMed] [Google Scholar]
- 19.Geier LM, Bekx MT, Connor EL. Factors contributing to initial weight loss among adolescents with polycystic ovary syndrome. Journal of pediatric and adolescent gynecology. 2012;25(6):367–370. [DOI] [PubMed] [Google Scholar]
- 20.Bekx MT, Connor EC, Allen DB. Characteristics of adolescents presenting to a multidisciplinary clinic for polycystic ovarian syndrome. Journal of pediatric and adolescent gynecology. 2010;23(1):7–10. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous Findings and Systemic Associations in Women With Polycystic Ovary Syndrome. JAMA Dermatol. 2016;152(4):391–398. [DOI] [PubMed] [Google Scholar]
- 22.Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism. 2013;98(12):4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002(246):1–190. [PubMed] [Google Scholar]
- 24.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism. 2011;96(7):1911–1930. [DOI] [PubMed] [Google Scholar]
- 26.Introduction: Standards of Medical Care in Diabetes—2020. Diabetes care. 2020;43(Supplement 1):S1–S2. [DOI] [PubMed] [Google Scholar]
- 27.Buyers E, Sass AE, Severn CD, Pyle L, Cree-Green M. Twelve-month Continuation of the Etonogestrel Implant in Adolescents With Polycystic Ovary Syndrome. Journal of pediatric and adolescent gynecology. 2020. [DOI] [PubMed] [Google Scholar]
- 28.Benson J, Severn C, Hudnut-Beumler J, et al. Depression in Girls With Obesity and Polycystic Ovary Syndrome and/or Type 2 Diabetes. Can J Diabetes. 2020;44(6):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonny AE, Appelbaum H, Connor EL, et al. Clinical variability in approaches to polycystic ovary syndrome. Journal of pediatric and adolescent gynecology. 2012;25(4):259–261. [DOI] [PubMed] [Google Scholar]
- 30.Sebastian MR, Wiemann CM, Bacha F, Alston Taylor SJ. Diagnostic Evaluation, Comorbidity Screening, and Treatment of Polycystic Ovary Syndrome in Adolescents in 3 Specialty Clinics. Journal of pediatric and adolescent gynecology. 2018;31(4):367–371. [DOI] [PubMed] [Google Scholar]
- 31.Baudson TG, Weber KE, Freund PA. More Than Only Skin Deep: Appearance Self-Concept Predicts Most of Secondary School Students’ Self-Esteem. Frontiers in psychology. 2016;7:1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vilar GN, Santos LA, Sobral Filho JF. Quality of life, self-esteem and psychosocial factors in adolescents with acne vulgaris. An Bras Dermatol. 2015;90(5):622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallerand IA, Lewinson RT, Parsons LM, et al. Risk of depression among patients with acne in the U.K.: a population-based cohort study. Br J Dermatol. 2018;178(3):e194–e195. [DOI] [PubMed] [Google Scholar]
- 34.Bulletins--Gynecology ACoP. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstetrics and gynecology. 2009;114(4):936–949. [DOI] [PubMed] [Google Scholar]
- 35.Altman M, Wilfley DE. Evidence update on the treatment of overweight and obesity in children and adolescents. J Clin Child Adolesc Psychol. 2015;44(4):521–537. [DOI] [PubMed] [Google Scholar]
- 36.Martin KA, Anderson RR, Chang RJ, et al. Evaluation and Treatment of Hirsutism in Premenopausal Women: An Endocrine Society Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism. 2018;103(4):1233–1257. [DOI] [PubMed] [Google Scholar]
- 37.Screening and Management of the Hyperandrogenic Adolescent: ACOG Committee Opinion, Number 789. Obstetrics and gynecology. 2019;134(4):e106–e114. [DOI] [PubMed] [Google Scholar]
- 38.S HP, Emily O, Amy S, Sarah B, Stephanie H, Melanie CG. Application of a Standard Cross-Specialty Workup for Diagnosis and Metabolic Screening of Obese Adolescents With Polycystic Ovary Syndrome. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2020. [DOI] [PubMed] [Google Scholar]