Abstract
Secondary metabolites from fungi, algae and lichens have remarkable biological activities as antibiotics, fungicides, antiviral drugs, and cancer therapeutics. This review focuses on the lichen-derived metabolite gyrophoric acid and other select secondary metabolites (e.g., usnic acid, salazinic acid, physodic acid, vulpinic acid ceratinalone, flavicansone, ramalin, physciosporin, tumidulin, atranorin, parmosidone) that modulate a number of cellular pathways relevant to several biomedical diseases and disorders, including cancer, diabetes and cardiovascular disease. We discuss the chemical structure and biochemical activities of gyrophoric acid and other compounds relative to the molecular mechanisms and cellular processes that these metabolites target in a distinct human and rodent cell types. The therapeutic promise of gyrophoric acid and similar lichen derived metabolites is associated with the chemical versatility of these compounds as polyaromatic depsides with functional carboxyl and hydroxyl side-groups that may permit selective interactions with distinct enzymatic active sites. Gyrophoric acid has been examined in a series of studies as an effective anticancer drug because it impinges on topoisomerase 1 activity, as well as causes cell cycle arrest, comprises cell survival, and promotes apoptosis. Because gyrophoric acid has cytostatic properties, its biological roles and possible medicinal utility may extend beyond effects on cancer cells and be relevant to any process that is controlled by cell growth and differentiation.
Keywords: Lichens, Gyrophoric Acid, Anticancer, Cardiovascular Diseases, Diabetes, Mechanism of Action, Secondary Metabolite, Depside, Topoisomerase, Proliferation, Apoptosis, Cancer Cytostatic, Cytotoxic
Introduction
Natural pharmacological compounds are used as therapies to treat a range of clinical conditions and disorders, including cancer. Many of these agents represent plant and fungus derived secondary metabolites such as polyphenols, such as flavonoids and phenolic acids [1–8]. A broad range of such secondary metabolites is produced by lichens, which represent a class of more than 10,000 symbiotic organisms consisting of fungi and photosynthetic algae [9] that are found in a wide variety of tropical and arctic environments with major differences in temperatures and sunlight [10]. More than 1,000 unique secondary metabolites including depsides, depsidones, dibenzofurans and chromones [11] are produced primarily by the fungal symbiont [12]. The considerable chemical diversity provides a major opportunity for the discovery of new compounds with different structural and cellular metabolic functions [13,14]. Unfractionated lichen extracts are bioactive against various microbial diseases such as bacterial, fungal and viral infections [15–20]. Production of secondary metabolites in lichens, which may enhance survival in ecologically diverse niches, may have evolved as part of biochemical defense mechanisms against other organisms such as bacteria and non-lichenized fungi [11,21–23].
This review discusses the secondary metabolite gyrophoric acid that has significant potential in medicinal applications. The purpose of this review paper is to provide an overview of current findings of gyrophoric acid, including its antitumor and antioxidant activities which render this compound attractive for applications as a chemotherapeutic agent to treat cancer cells, as well as inflammation and glycation in diabetes and cardiovascular disease. We also discuss published studies that focus on the mode of action of this compound in different cell types and physiological processes. These studies indicate that gyrophoric acid affects molecular mechanisms related to proliferation, apoptosis and enzyme inhibitory effects in different biological contexts. We discuss specific studies on the potential functions and mechanisms of action of gyrophoric acid in different biological contexts in greater detail. These studies provide the foundation for our current understanding of the utility of gyrophoric acid as a lead compound for further drug development and possible therapeutic applications. Although a full picture of its function has not yet emerged, considerable progress has been made that warrants further investigation.
Biomedical roles of lichen-derived secondary metabolites in medicine
Gyrophoric acid has been characterized in an extensive number of studies on the use of lichens as traditional medicine. It is one of many distinct metabolites present in lichens that has strong antimicrobial activities against several bacteria and fungi among which are human pathogens [24]. Gyrophoric acid and other lichen derived compounds have been investigated for possible use in anticancer therapies and inflammatory diseases based on cytostatic and cytotoxic effects. For example, lichen-derived secondary metabolites exert anti-proliferative effects in a variety of tumor cell types (e.g., breast, lung, melanoma and colon carcinomas) by modulating cell cycle progression, cell survival and/or cell death with concomitant alterations in distinct cell signaling pathways and changes in gene expression, based on in vitro studies using cell culture models and enzymes [25–34]. Beyond potential utility as cancer therapeutics, lichen extracts and purified lichen-derived metabolites have also possible uses for the treatment of cardiovascular diseases, asthma, pulmonary inflammation and gastrointestinal disorders [35–37]. Results on the biological activities of lichen extracts and metabolites have been sufficiently encouraging to permit further bio-screening of new lichen-derived natural products.
Gyrophoric acid as a paradigm for bioactive polyphenolic compounds from lichen species
Gyrophoric acid is a prevalent secondary metabolite in lichens [38] (Figure 1). It has been detected in 31 out of 33 lichen species within the Umbilicaria genus [22], including Umbilicaria muhlenbergii, which is a common lichen species in North America. Gyrophoric acid is a polyphenolic depside that is characterized by three monocyclic aromatic rings [21] (Figure 1A). The aromatic rings in these phenolic compounds permit scavenging of free radicals consistent with a general anti-oxidant function. In addition, these aromatic rings provide a versatile scaffold that together with additional hydroxyl and methyl groups can dock with (and block) active sites within different enzymes and receptors [15]. This biochemical versatility of gyrophoric acid and other depsides permits consideration of their bioactivities in a range of biological activities [39,40].
Figure 1: Overview of biochemical activities of gyrophoric acid.
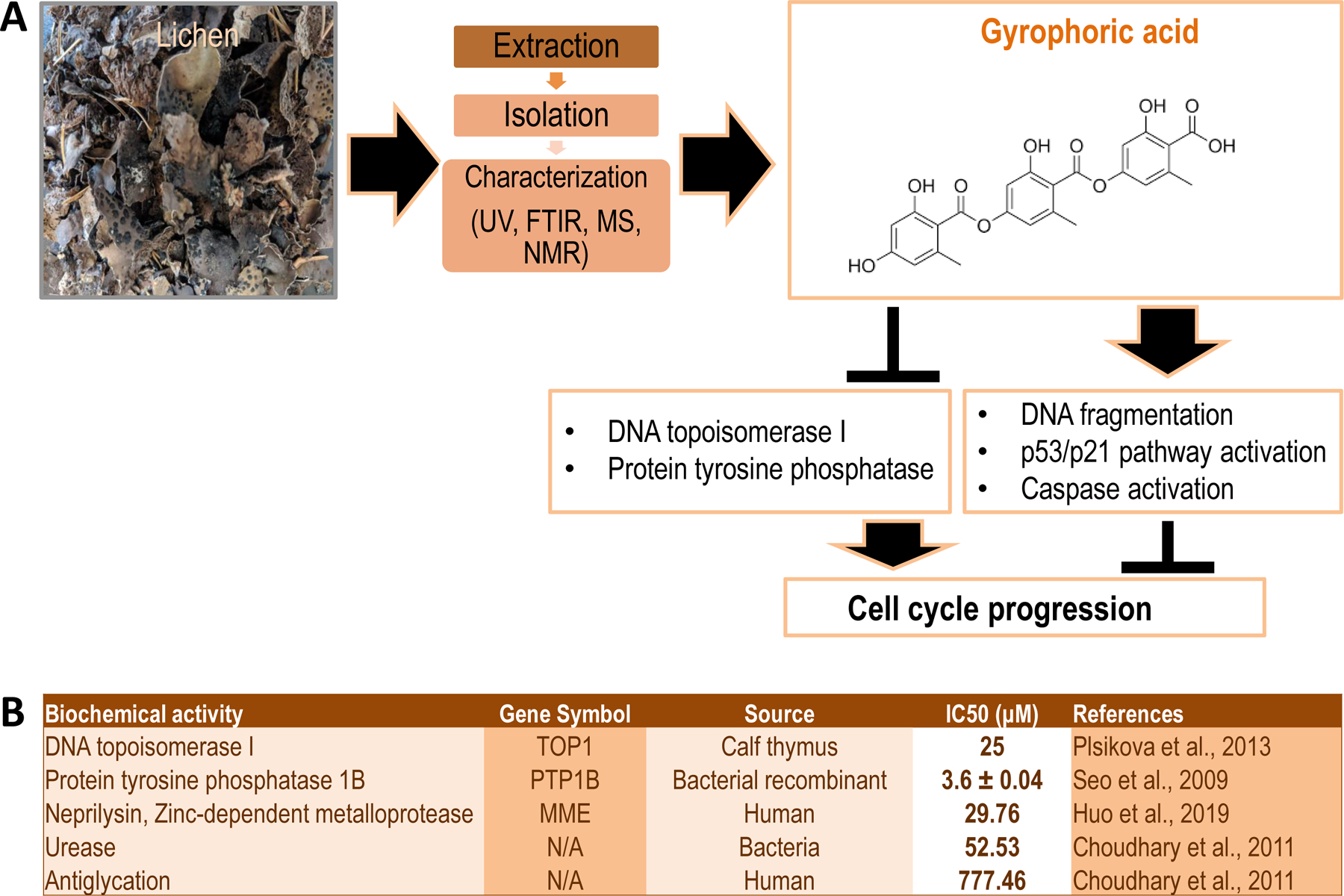
(A) Image of Umbilicaria muhlenbergii, a lichen species that is typically used for the extraction and isolation of secondary metabolites. These metabolites are chemically characterized by ultraviolet (UV), Fourier-transform infrared (FTIR), mass spectroscopy (MS), and nuclear magnetic resonance (NMR) spectroscopy. One of these secondary metabolites is gyrophoric acid, which is a polyaromatic compound with carboxyl and hydroxyl side chains that inhibits DNA topoisomerase and protein tyrosine phosphatase activities, while promoting DNA fragmentation, activation of the cell protective p53/p21 pathway and the proteolytic caspase cascade. (B) Tabular summary of studies that have examined the biochemical effects of gyrophoric acid on different types of enzymes (column 1), gene symbols of enzymes insofar appropriate (column 2), species or sources of the protein (column 3), the half-maximum inhibitory concentration (IC50) (column 4) and relevant references (column 5).
Biological effects of gyrophoric acid on distinct cell types and biochemical effects on diverse enzymes
Gyrophoric acid may have evolved perhaps as a potential antimicrobial agent for non-lichen species based on its bioactivity as a cytotoxic or cytostatic agent in distinct eukaryotic cell types. Indeed, our current understanding of gyrophoric acid is that it suppresses mammalian cell cycle progression by blocking Topoisomerase 1 activity (encoded by the TOP1 gene). This inhibition is therefore expected to generate DNA strand breaks and activate the p53/p21 DNA damage pathway (encoded by the TP53 and CDKN1A genes, respectively) (Figure 1A). The resulting cell cycle arrest may occur concomitant with caspase activation and apoptosis if the genome is degraded to the point where it cannot be properly repaired.
Gyrophoric acid not only inhibits Topoisomerase I but also targets other enzymes including eukaryotic protein tyrosine phosphatases (e.g., PTP1B), the zinc-dependent metalloprotease neprilysin (i.e., MME), bacterial urease and enzymes that control glycation (Figure 1B). Several studies have defined the inhibitory concentration at which gyrophoric acid inhibits various enzymes from different biological sources (Fig. 1B). These studies revealed that the protein tyrosine phosphatase PTP1B is the most sensitive target with an IC50 below 5µM, while the half maximum inhibitory concentration for TOP1 is considerably higher (IC50>35µM) and yet even higher for other enzymes that have been studied [41] (Fig. 1B). Notably, enzymes targeted by gyrophoric acid are broadly conserved in eukaryotic species including fungi and yeast. Based on the phylogenetic conservation of its putative molecular targets, gyrophoric acid is predicted to have biological effects in organisms and cells ranging from yeast to man.
The biological effects of gyrophoric acid have also been investigated in a variety of distinct cell types in a dose and time dependent manner (Figure 2). The majority of studies have been performed with different tumor derived cell types (e.g., K562 and HL60 leukemia cells, A375 and A2780 ovarian cancer cells, HT29 and MCF7 breast cancer cells and HeLa cervical cancer cells). Additionally, gyrophoric acid has been tested in a very limited number of non-tumor cell types, such as immortalized Jurkat T lymphocytes, lymphocytes, keratinocytes and hepatocytes (Fig. 2). Our group has recently expanded these studies to include U2OS osteosarcoma cells and normal human mesenchymal stem cells (MSCs) derived from the perivascular stroma of adipose tissue (unpublished observations). Collectively, these studies reveal that different cells exhibit distinct sensitivities to different concentrations of gyrophoric acid in a variety of biological assays that examined the cellular mechanisms of action based on its versatile biochemical inhibitory activities. The overall conclusion is that gyrophoric acid affects human cell types within a fairly broad concentration range and has biological effects with micromolar doses between 50 and 500µM (Fig. 2). This concentration range is quite high and could suggest that gyrophoric acid does not target one or few specific enzymes with high affinity, but rather many enzymes with low affinity. The latter concept would be consistent with the idea that lichens generate gyrophoric acid as a protective pleiotropic compound that targets multiple conserved essential enzymes in other eukaryotic and microbial species.
Figure 2: Overview of cellular activities of gyrophoric acid.
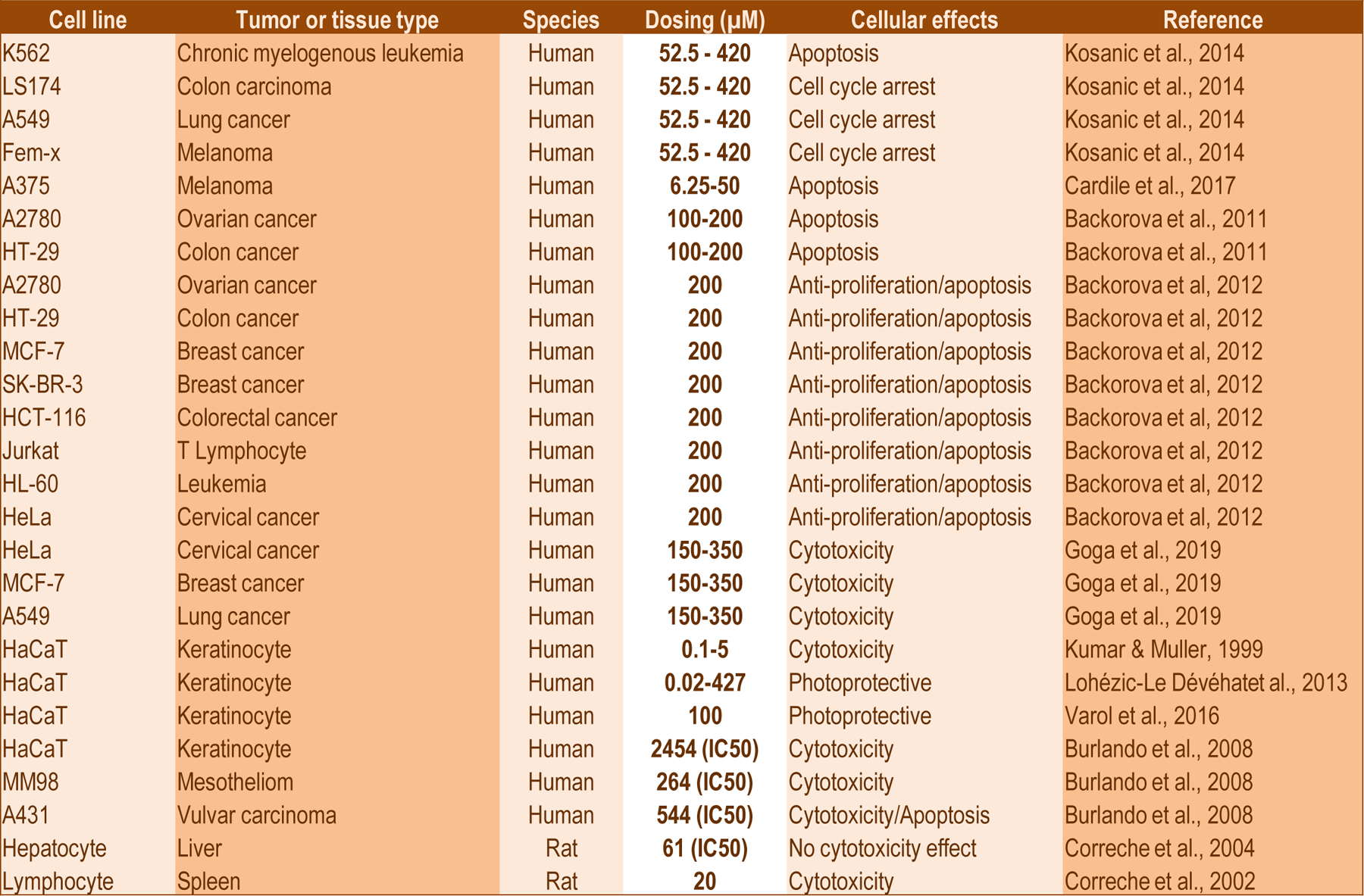
The image shows a tabular summary of tumor cell lines and normal primary cell types (column 1), the biological origin (column 2) and species of the cells (column 3), the range of gyrophoric acid concentrations that were tested (column 4), cellular processes affected by gyrophoric acid (column 5) and relevant references (column 6).
Cytostatic effects of gyrophoric acid on tumor cell lines
The cell cycle in proliferating tumor cells is controlled by checkpoints that ensure replication of the genome during S phase and proper separation of the two daughter cells during the mitotic process of cell division [42]. Deregulation in cell cycle progression dysregulates proliferation of cells resulting in the unconstrained growth observed in tumor cells [43]. Many natural compounds produced by plants and lichens exhibit the ability to inhibit cancer cell proliferation by controlling cell cycle progression during G1, S and G2 phases or mitosis, and any such compounds could potentially be considered for chemotherapeutic strategies [44]. Gyrophoric acid has clear effects on the p53/p21 (TP53/CDKN1A) pathway as discussed above in different cancer cell types. For example, Fem-X and K562 cell lines exhibit apoptosis after exposure to gyrophoric acid as reflected by increased numbers of cells in the sub-G1 phase and decreased percentages of cells in G0/G1 and S/G2/M phases [12]. Gyrophoric acid is also known to exert inhibitory effects on proliferation by provoking cell cycle arrest at the quiescence related checkpoints at G0/G1 in A2780, HCT-116-p53+/+, HCT-116-p53−/− and HL-60 cells [45]. In addition, gyrophoric acid triggers a cell cycle arrest in S phase in both HT-29 and A2780 cells [29], while causing a G0/G1 arrest in HeLa cells under different treatment conditions. Consistent with the cytostatic effects of gyrophoric acid, this compound inhibits colony formation in SK-BR-3 breast cancer cells, even though this result was not replicated in HT-29 and HCT-116 colon carcinomas or A2780 ovarian carcinoma cells [45]. Thus, gyrophoric acid has pleiotropic and inhibitory effects on cell cycle progression in a broad range of tumor cell types. These effects depend on the tumor type, perhaps because the pharmacokinetics of gyrophoric acid may differ in different cell types. For example, the potency and bioavailability of gyrophoric acid may differ in different cell types due to conversion of gyrophoric acid by cytochrome P450 monooxygenases that target xenobiotics, removal of gyrophoric acid by ATP sodium-potassium exchange pumps, or yet other cellular mechanisms.
Beyond cell cycle effects, gyrophoric acid also may affect oxidative processes and cell metabolism. One study focused on the role of gyrophoric acid as an antioxidant that provides UV protection in skin-related melanoma cells [46]. In addition, A375 melanoma cells treated with gyrophoric acid show induction of lactate dehydrogenase which is secreted into the culture media as an indicator of cytotoxicity [47], although no release was observed in hepatocyte cells [48]. When exposed to gyrophoric acid, the keratinocytic HaCaT cell line also releases lactates dehydrogenase into media as a marker of cellular damage [49]. These effects on HaCaT keratinocytes are primarily cytostatic rather than cytotoxic in nature [50]. Effective concentrations of gyrophoric acid that decrease proliferation also affect cell metabolic activity of HeLa cells, MCF-7 and A549 cells [14]. These metabolic effects of gyrophoric acid could be direct by interfering with one or more uncharacterized enzymes that have key metabolic functions, or perhaps be the secondary consequence of reduced energy requirements that accompany a block in cell cycle progression.
Apoptosis related effects of gyrophoric acid
Gyrophoric acid is known to trigger programmed cell death. Apoptosis of cells damaged by this compound occurs concomitant with changes in nuclear morphology (‘blebbing’), breakdown of cellular components and production of paracrine signals that recruit macrophages. Gyrophoric acid may in principle cause apoptosis via intrinsic or extrinsic apoptotic pathways that involve different networks of proteins (Figure 3). The intrinsic pathway is associated with mitochondria and is linked to the ratio between mitochondrial BCL2 proteins and the antagonist BAX proteins that together control cytochrome C retention [42]. This intrinsic pathway is regulated by the nuclear transcription factor p53/TP53, which is activated by DNA damage. Increased levels of BAX and/or reduced levels of BCL2 activate death signals in mitochondria and release cytochrome C into the cytoplasm. Extrinsic receptor mediated pathways are initiated by the binding of pro-apoptotic ligands (e.g., TNF-α) to their cognate cell death receptors (e.g., the TNF-α receptor TNFR1/TNFRSF1A). Activation of cell death receptors induces caspases that represent key proteases mediating apoptosis [51]. It has been established that natural lichen derived metabolites may promote formation of reactive oxygen species (ROS) as part of an apoptotic process that (i) decreases mitochondrial membrane potential, (ii) decreases the BCL2/BAX ratio and (iii) increases the activity of the caspase cascade (i.e., CASP3, CASP8 and CASP9) [52–57]. These events ultimately promote DNA fragmentation and disintegration of cells. Hence, gyrophoric acid may have a significant role in both intrinsic and extrinsic apoptotic pathways. Many different cancer cell types have been investigated for responsiveness to gyrophoric acid at different doses and multiple time points (Figure 2). Apoptotic effects of gyrophoric acid are observed between 50 to 200µM as reflected by upregulation of established markers of apoptosis, such as ROS induction and caspase pathways (Figure 2). The ability of gyrophoric acid to induce programmed cell death is consistent with its promising potential as an apoptosis-inducing agent that can be used to decrease tumor cells.
Figure 3:
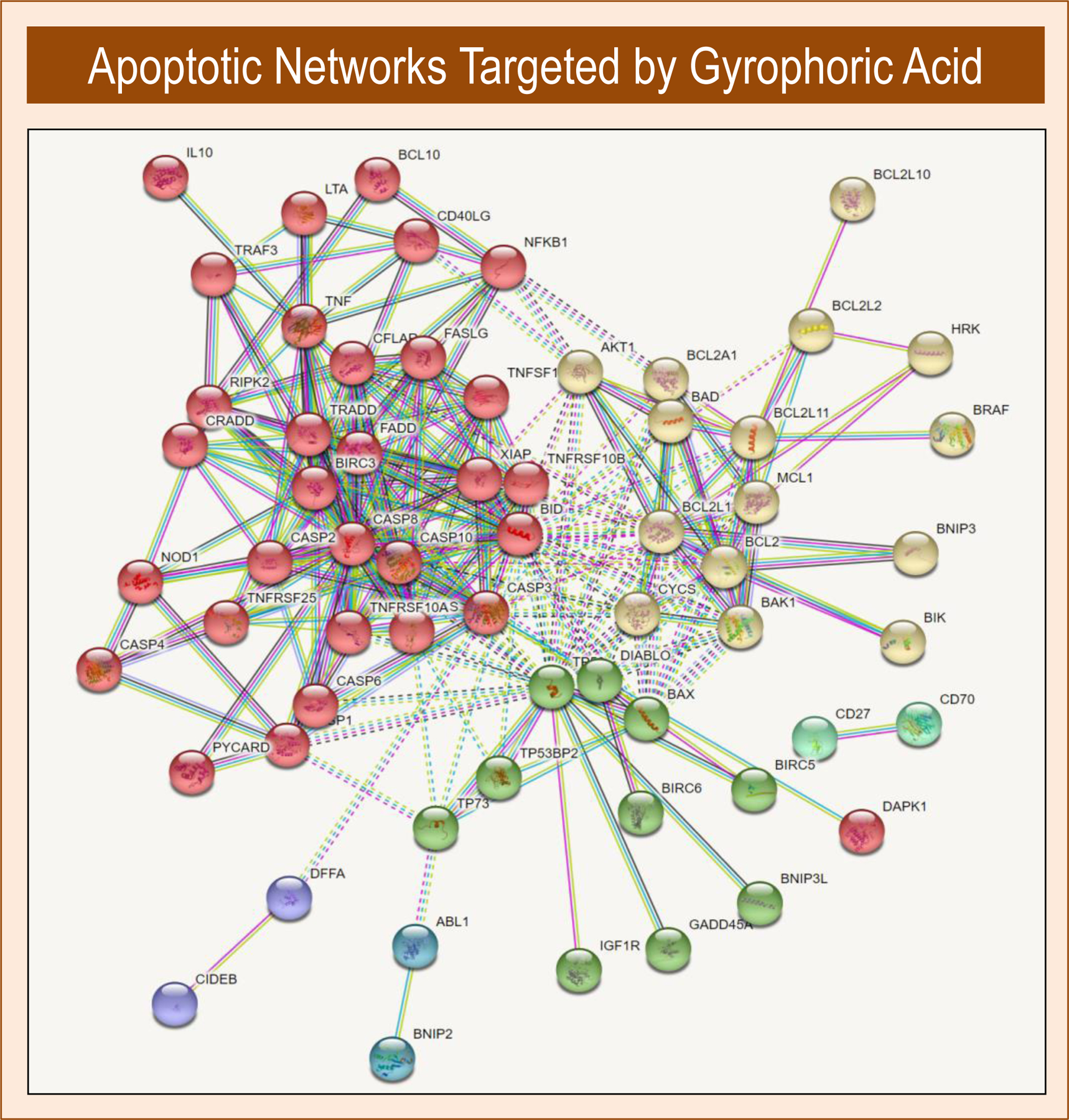
Diagram of apoptotic pathways activated by gyrophoric acid. The protein network shows anti-apoptotic and pro-apoptotic proteins known to be involved in induction of apoptosis, including proteins that respond to intracellular DNA damage and repair or extracellular apoptotic signals such as death domain receptors. The protein network was generated using proteins known to be involved in apoptosis based on gene ontology terms. String v11.0 was used to render a protein network using the highest stringency settings with removal of unconnected proteins.
Enzyme inhibitory effect of gyrophoric acid in diabetes and chronic heart failure
Beyond the bioactivity of gyrophoric acid as a potential anti-cancer agent, this compound also may have important medicinal potential by inhibiting glycation related enzymes and enzyme inhibitors [58]. The clinical significance of these findings is that many age-related diseases are related to glycation including diabetes, kidney diseases, inflammatory processes and other degenerative disorders (e.g., atherosclerosis, osteoporosis, neurodegenerative disease) [59]. Consistent with these studies, gyrophoric acid may be effective in diabetes [31,58,60] and chronic heart disease [61]. Anti-diabetic therapies could be developed based on the ability of gyrophoric acid to inhibit diabetes related enzymes as α-amylase [58] phosphatase PTP1B [41], and enzymes supporting glycation. Lichen species and derived metabolites like gyrophoric acid decrease glycation and reduce pathological parameters of diabetes which may be related to the versatile aromatic structures and associated functional groups of gyrophoric acid (e.g., carboxy and hydroxyl groups) that permit binding to different types of active sites in distinct enzymes [62] (summarized in Figure 1). In vitro screening has revealed that gyrophoric acid has antiglycation activity on bovine serum albumin and inhibits the microbial enzyme urease. The inhibitory effects of gyrophoric acid on PTP1B may be related to the role of this intracellular phosphatase in suppression of insulin production [63]. The inhibitory effects of gyrophoric acid on PTP1B and glycation suggest that this compound could be considered for potential pharmacotherapies to decrease the clinical complications of diabetes. Beyond gyrophoric acid, other studies have examined the inhibitory effect of lichen substances on α-glucosidase, an enzyme that converts glycogen and disaccharides to α-glucose. Two of these studies focused on the depsidone, parmosidone, and new meta-depsidone, parmosidone K, from the lichen Parmotrema tsavoense which revealed that these compounds inhibit glucosidase activity within the micromolar range (respectively, IC50 values in the range of 10.7 to 17.6 μM [64] and 3.12 µM) [65].
The combined knowledge of traditional and modern pharmacology has led to consideration of gyrophoric acid as a novel potential therapeutic for heart disease. Multi-target inhibitory modeling was performed in silico to assess new strategies for reducing pathological manifestations of chronic heart failure. In one study, gyrophoric acid was tested for its ability to block the activity of angiotensin II type 1 receptor (AT1/AGTR1), which regulates blood pressure in the cardiovascular system together with the kidney related protease neprilysin (NEP) [61]. Improvements in cardiovascular disease and death due to heart failure can be achieved by combination treatment that targets both AGTR1 and NEP. Multi-drug target modeling studies were performed to suggest that gyrophoric acid may directly interact with AGTR1, while subsequent biological screening revealed that gyrophoric acid may antagonize AGTR1 as predicted [61]. Hence, gyrophoric acid may have potential in cardiovascular disease and this interesting suggestion warrants further exploration.
Beyond gyrophoric acid: biological effects of other lichen-derived compounds
Apart from gyrophoric acid as a prominent secondary metabolite, drug discovery searches with lichen extracts have results in identification of other lichen-derived chemical entities with antioxidant, anti-cancer and anti-inflammatory agents. For example, usnic acid is a dibenzofuran compound that has been isolated from extracts of the lichen Usnea barbata [30,66], Niebla homalea and other lichen species and has demonstrated activity against cancer cell proliferation. Usnic acid has modest antiproliferative effects in A2780 ovarian and MCF7 breast cancer cell lines in the micromolar range (IC50 values of, respectively, 3.8 μM and 6.8 μM) [67]. Mechanistically, this compound induces apoptosis in human lung cancer cells in a time and concentration dependent manner via effects on mitochondrial membrane depolarization [68]. In addition, usnic acid deregulates the cytoprotective p53 (TP53) gene and the pro-apoptotic genes BCL2 and BAX in both non-malignant (Vero, L929) and cancer-derived (CaCo2, RD, Hep2C, HepG2, Wehi) cell lines [55]. Similar mechanisms were examined in human HCT116 colorectal cancer cells where usnic acid suppresses BCL2-related antiapoptotic genes (e.g., MCL1), while inducing apoptotic genes (e.g., BAX, TP53, CDKN1A/p21) in a concentration-dependent manner [69]. Usnic acid is also known to inhibit mTOR/MTOR activity by suppressing protein kinase C alpha type (PKC-A/ PRKCA) and increasing LDH release in human colorectal cancer (HCT116, LS174) cells [70]. Furthermore, this compound mediates anti-proliferative effects via miRNA dependent mechanisms that target Hedgehog, TGF- and MAPK apoptosis pathways in human breast cancer cell lines (MDA-MB-231, BT-474, and MCF7 cells) [71], while synergizing with tamoxifen to suppress proliferation of MCF7 cells [72]. Usnic acid also suppresses proliferation of both normal fibroblasts (V79) and oral squamous carcinoma cells (CAL-27) [30], as well as cervical cancer cells by inhibiting PD-L1 expression and enhancing T-lymphocyte activity towards tumors [73]. This compound also reduced cell survival and increased programmed cell death in LUSC lung carcinoma cells by reactive oxygen species (ROS) produced by mitochondrial respiratory chain, which then reduces NRF2 stability through deregulation of the PI3K/AKT pathway [69].
The number of lichen species that has been examined for bioactive compounds that potential medicinal properties in blocking cancer cell growth continues to expand rapidly. Apart from gyrophoric acid and usnic acid, many other potential anti-proliferative compounds have been extracted from lichens, including salazinic acid, physodic acid, and tumidulin (from Niebla homalea [67,74] or Pseudevernia furfuracea [75]), ceratinalone (a depsidone secondary metabolite from Usnea ceratina) [64], flavicansone (a depsidone from Teloschistes flavicans) [76], uncharacterized methanol-extractable compounds (from Physconia hokkaidensis) [56], diphenyl ethers lichen-derived praesorethers E, F and G [77], as well as a series of identified secondary metabolites such as vulpinic acid [78], atranorin [79], ramalin [80], and physciosporin [81]. Similar to the many studies focusing on gyrophoric acid, these lichen crude extracts or their purified metabolites were investigated for apoptotic and anti-proliferative effects in a wide variety of different cancer cell lines. Two systematic studies examined the antitumor activities of extracts from a panels containing about a dozen distinct lichen species [29,82]
Each of these compounds and extracts was examined for cytotoxic and cytostatic properties in many different cancer cell lines, similar to studies on the molecular mechanism of gyrophoric action discussed above. The list of cancer cell lines that were tested for sensitivity to lichen compounds were derived from patients with colorectal and stomach tumors (DLD-1, HCT116 and AGS), hematopoietic cancer (HL-60), lung cancer (A549), cervical carcinoma (HeLa), hepatoma (HepG2), and breast cancer (MDA-MB-231 and MCF-7) [32,56]. Similar to gyrophoric acid, these studies revealed that lichen derived compounds can increase p53 expression [78], as well as suppress pro-proliferative WNT/β-catenin (CTNNB1) and AP1 (FOS/JUN) related pathways that control cell growth regulatory factors such as cyclin-D1 and c-myc [31]. In addition, several of these studies also revealed that lichen extracts or compounds can trigger detoxification and antioxidant defense mechanisms involving transcription factor NRF2 and its downstream target genes, while suppressing inflammation related pathways (e.g., NFKB1 and STAT3) [32] and the TGF-β (TGFB1/SMAD2/SMAD3) pathway [75].
While studies on lichens extracts and/or isolated lichen metabolites has provided insight into their potential use as anticancer agents, clinical investigations will be required to obtain a comprehensive understanding of these lichen derived compounds in cancer patients. The current body of work on lichen species studies is definitely very encouraging and indicates that compounds from lichen species are quite adaptable and may be useful for potential therapeutic strategies for cancer treatment in the clinic.
Conclusions
The general conclusion that is apparent from all studies on gyrophoric acid is that this secondary metabolite from lichens is an extraordinarily versatile polyaromatic depside that has broad biological activity in a number of biological contexts. The compound has been most often studied as an inhibitory agent that constrains tumor growth by blocking topoisomerase I activity, inducing p53-dependent DNA damage pathways and provoking apoptosis networks including the proteolytic caspase cascade (Figure 3). Beyond these anti-tumor effects, gyrophoric acid may also affect enzymes and pathways related to diabetes and cardiovascular disease. The biological versatility gyrophoric acid is linked to its ability to target and inhibit multiple different enzymes at micromolar concentrations. This ability to bind many targets is based on its unique chemical structure that contains a polyaromatic scaffold with functional side groups that permit interactions with diverse active sites. It can certainly not be ruled out that gyrophoric acid may target additional enzymes that have not yet been examined. While several potential mechanisms of action for gyrophoric acid have been described, these inhibitory activities must be further defined and refined. Another interesting concept is the development of drug combination of the secondary metabolite gyrophoric acid with other lichen isolated metabolites or conventional chemotherapeutic drugs. Combination therapies could be considerably more effective compared to treatment with single anticancer compounds. Furthermore, lichen-derived anticancer substances could be combined with small molecules that affect genetic and epigenetic pathways, including DNA methylation, histone modification and non-coding RNA expression (e.g., miRNAs and LNCRNAs). Therefore, further investigations at the biochemical, molecular, cellular and biological levels in both in vitro and in vivo models will undoubtedly increase our understanding of the biological actions of this promising agent in drug design and pharmacotherapies for a broad range of medical conditions.
Highlights.
Lichen-derived metabolites inhibit clinically relevant cellular pathways
Gyrophoric acid targets many distinct enzymes with low affinity
Gyrophoric acid has cytostatic effects by blocking key cellular pathways
Cytostatic effects of gyrophoric acid may have use in cancer treatment
Acknowledgments
We thank Roman Thaler, Amel Dudakovic and Noelle Larson for stimulating discussions.
Funding
This work was supported by Mitacs (#FR37889)(to MM) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR049069 to AvW). We also thank William and Karen Eby for generous philanthropic support.
Abbreviations:
- AGTR1
Angiotensin II Receptor Type 1
- AT1
Angiotensin II Type 1 Receptor
- ATP
Adenosine Triphosphat
- BAX
BCL2 Associated X, Apoptosis Regulator
- BCL2
B-Cell CLL/Lymphoma 2 Apoptosis Regulator
- CASP3
Caspase 3
- CASP8
Caspase 8
- CASP9
Caspase 9
- CDKN1A
Cyclin Dependent Kinase Inhibitor 1A
- CHF
Congestive Heart Failure
- DNA
Deoxyribonucleic Acid
- FTIR
Fourier-Transform Infrared
- IC
Inhibitory Concentration
- MME
Membrane Metalloendopeptidase
- MS
Mass
- MSC
Mesenchymal stem cell
- NEP
Neutral Endopeptidase
- NMR
Nuclear Magnetic Resonance
- PARP1
Poly(ADP-Ribose) Polymerase 1
- PARP2
Poly(ADP-Ribose) Polymerase 2
- PARP3
Poly(ADP-Ribose) Polymerase 3
- PTP1B
Protein-Tyrosine Phosphatase 1B
- ROS
Reactive Oxygen-Containing Species
- TNF
Tumor Necrosis Factor
- TNFRSF1A
TNF Receptor Superfamily Member 1A
- TOP1
DNA Topoisomerase I
- TP53
Tumor Protein P53
- UV
Ultraviolet
- μM
Micromolar
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Safe S, Jayaraman A, Chapkin RS, Howard M, Mohankumar K, Shrestha R, Flavonoids: structurefunction and mechanisms of action and opportunities for drug development., Toxicol. Res 37 (2021) 147–162. 10.1007/s43188-020-00080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang S, Yuan X-H, Wang S-Q, Zhao W, Chen X-B, Yu B, FDA-approved pyrimidine-fused bicyclic heterocycles for cancer therapy: Synthesis and clinical application., Eur. J. Med. Chem 214 (2021) 113218. 10.1016/j.ejmech.2021.113218. [DOI] [PubMed] [Google Scholar]
- [3].Sansone C, Bruno A, Piscitelli C, Baci D, Fontana A, Brunet C, Noonan DM, Albini A, Natural Compounds of Marine Origin as Inducers of Immunogenic Cell Death (ICD): Potential Role for Cancer Interception and Therapy., Cells 10 (2021). 10.3390/cells10020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Garcia-Oliveira P, Otero P, Pereira AG, Chamorro F, Carpena M, Echave J, Fraga-Corral M, Simal-Gandara J, Prieto MA, Status and challenges of plant-anticancer compounds in cancer treatment, Pharmaceuticals 14 (2021) 1–28. 10.3390/ph14020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dehelean CA, Marcovici I, Soica C, Mioc M, Coricovac D, Iurciuc S, Cretu OM, Pinzaru I, Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy., Molecules 26 (2021). 10.3390/molecules26041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aaghaz S, Sharma K, Jain R, Kamal A, β-Carbolines as potential anticancer agents., Eur. J. Med. Chem 216 (2021) 113321. 10.1016/j.ejmech.2021.113321. [DOI] [PubMed] [Google Scholar]
- [7].Kluska M, Woźniak K, Natural Polyphenols as Modulators of Etoposide Anti-Cancer Activity., Int. J. Mol. Sci 22 (2021). 10.3390/ijms22126602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].El Omari N, Bakha M, Imtara H, Guaouguaoua F-E, Balahbib A, Zengin G, Bouyahya A, Anticancer mechanisms of phytochemical compounds: focusing on epigenetic targets., Environ. Sci. Pollut. Res. Int 28 (2021) 47869–47903. 10.1007/s11356-021-15594-8. [DOI] [PubMed] [Google Scholar]
- [9].Sipman HJM, Aptroot A, Where are the missing lichens?, Mycol. Res (2001). 10.1017/S0953756201004932. [DOI] [Google Scholar]
- [10].Melo MGD, dos Santos JPA, Serafini MR, Caregnato FF, de Bittencourt Pasquali MA, Rabelo TK, da Rocha RF, Quintans L, de Souza Araújo AA, da Silva FA, Moreira JCF, Gelain DP, Redox properties and cytoprotective actions of atranorin, a lichen secondary metabolite, Toxicol. Vitr 25 (2011) 462–468. 10.1016/j.tiv.2010.11.014. [DOI] [PubMed] [Google Scholar]
- [11].Calcott MJ, Ackerley DF, Knight A, Keyzers RA, Owen JG, Secondary metabolism in the lichen symbiosis, Chem. Soc. Rev 47 (2018) 1730–1760. 10.1039/c7cs00431a. [DOI] [PubMed] [Google Scholar]
- [12].Kosanić M, Ranković B, Stanojković T, Rančić A, Manojlović N, Cladonia lichens and their major metabolites as possible natural antioxidant, antimicrobial and anticancer agents, LWT - Food Sci. Technol 59 (2014) 518–525. 10.1016/j.lwt.2014.04.047. [DOI] [Google Scholar]
- [13].Ristic S, Rankovic B, Kosanić M, Stamenkovic S, Stanojković T, Sovrlić M, Manojlović N, Biopharmaceutical Potential of Two Ramalina Lichens and their Metabolites, Curr. Pharm. Biotechnol 17 (2016) 651–658. 10.2174/1389201017666160401144825. [DOI] [PubMed] [Google Scholar]
- [14].Goga M, Kello M, Vilkova M, Petrova K, Backor M, Adlassnig W, Lang I, Oxidative stress mediated by gyrophoric acid from the lichen Umbilicaria hirsuta affected apoptosis and stress/survival pathways in HeLa cells, BMC Complement. Altern. Med 19 (2019) 221. 10.1186/s12906-019-2631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Manojlovic NT, Vasiljevic PJ, Maskovic PZ, Juskovic M, Bogdanovic-Dusanovic G, Chemical composition, antioxidant, and antimicrobial activities of lichen Umbilicaria cylindrica (L.) delise (Umbilicariaceae), Evidence-Based Complement. Altern. Med 2012 (2012). 10.1155/2012/452431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Basile A, Rigano D, Loppi S, Di Santi A, Nebbioso A, Sorbo S, Conte B, Paoli L, De Ruberto F, Molinari AM, Altucci L, Bontempo P, Antiproliferative, antibacterial and antifungal activity of the lichen Xanthoria parietina and its secondary metabolite parietin, Int. J. Mol. Sci 16 (2015) 7861–7875. 10.3390/ijms16047861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gonçalves JP, Martins MCB, Buril M. de L.L., Aguiar JDS, Da Silva TG, Dos Santos Souza TG, Santos NPDS, Chagas CA, Pereira EC, Falcão EPDS, Da Silva NH, Antineoplastic Activity and Genotoxicity of Organic Extracts and Barbatic Acid Isolated from the Lichen Cladonia salzmannii Nyl, Int. Arch. Med 11 (2018). 10.3823/2594. [DOI] [Google Scholar]
- [18].Goncu B, Sevgi E, Kizilarslan Hancer C, Gokay G, Ozten N, Differential anti-proliferative and apoptotic effects of lichen species on human prostate carcinoma cells., PLoS One 15 (2020) e0238303. 10.1371/journal.pone.0238303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Aoussar N, Laasri FE, Bourhia M, Manoljovic N, Mhand RA, Rhallabi N, Ullah R, Shahat AA, Noman OM, Nasr FA, Almarfadi OM, El Mzibri M, Vasiljević P, Benbacer L, Mellouki F, Phytochemical Analysis, Cytotoxic, Antioxidant, and Antibacterial Activities of Lichens, Evidence-Based Complement. Altern. Med 2020 (2020) 8104538. 10.1155/2020/8104538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cuong T, Thoa N, Bioactive Compounds from Lichens as Promising Biomaterial for the Treatment of Influenza Virus: A Review, J. Sci. Res. Reports 18 (2018) 1–15. 10.9734/jsrr/2018/39899. [DOI] [Google Scholar]
- [21].Goga M, Elečko J, Marcinčinová M, Ručová D, Bačkorová M, Bačkor M, Lichen Metabolites: An Overview of Some Secondary Metabolites and Their Biological Potential, in: 2018: pp. 1–36. 10.1007/978-3-319-76887-8_57-1. [DOI] [Google Scholar]
- [22].Posner B, Feige GB, Huneck S, Studies on the chemistry of the lichen genus umbilicaria hoffm, Zeitschrift Fur Naturforsch. - Sect. C J. Biosci 47 (1992) 1–9. 10.1515/znc-1992-1-202. [DOI] [Google Scholar]
- [23].Alim A, Goze I, Goze HM, Tepe B, Serkedjieva J, In vitro antimicrobial and antiviral activities of the essential oil and various extracts of Salvia cedronella Boiss, J. Med. Plants Res 3 (2009) 413–419. [Google Scholar]
- [24].Ranković B, Mišić M, Sukdolak S, The antimicrobial activity of substances derived from the lichens Physcia aipolia , Umbilicaria polyphylla, Parmelia caperata and Hypogymnia physodes, World J. Microbiol. Biotechnol 24 (2008) 1239–1242. 10.1007/s11274-007-9580-7. [DOI] [Google Scholar]
- [25].Ari F, Ulukaya E, Oran S, Celikler S, Ozturk S, Ozel MZ, Promising anticancer activity of a lichen, Parmelia sulcata Taylor, against breast cancer cell lines and genotoxic effect on human lymphocytes, Cytotechnology 67 (2015) 531–543. 10.1007/s10616-014-9713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Şekerli M, Kılıç N, Cansaran Duman D, The Molecular Mechanisms of the effect of anticancer activity on lichen metabolites, Turkish Bull. Hyg. Exp. Biol 74 (2017) 95–102. 10.5505/turkhijyen.2016.24650. [DOI] [Google Scholar]
- [27].Paluszczak J, Kleszcz R, Studzińska-Sroka E, Krajka-Kuźniak V, Lichen-derived caperatic acid and physodic acid inhibit Wnt signaling in colorectal cancer cells, Mol. Cell. Biochem 441 (2018) 109–124. 10.1007/s11010-017-3178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cardile V, Graziano ACE, Avola R, Piovano M, Russo A, Potential anticancer activity of lichen secondary metabolite physodic acid, Chem. Biol. Interact 263 (2017) 36–45. 10.1016/j.cbi.2016.12.007. [DOI] [PubMed] [Google Scholar]
- [29].Bačkorová M, Jendželovský R, Kello M, Bačkor M, Mikeš J, Fedoročko P, Lichen secondary metabolites are responsible for induction of apoptosis in HT-29 and A2780 human cancer cell lines, Toxicol. Vitr 26 (2012) 462–468. 10.1016/j.tiv.2012.01.017. [DOI] [PubMed] [Google Scholar]
- [30].Popovici V, Matei E, Cozaru GC, Aschie M, Bucur L, Rambu D, Costache T, Cucolea IE, Vochita G, Gherghel D, Caraiane A, Badea V, Usnic Acid and Usnea barbata (L.) F.H. Wigg. Dry Extracts Promote Apoptosis and DNA Damage in Human Blood Cells through Enhancing ROS Levels., Antioxidants (Basel, Switzerland) 10 (2021). 10.3390/antiox10081171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhou R, Yang Y, Park S, Nguyen TT, Seo Y, Lee KH, The lichen secondary metabolite atranorin suppresses lung cancer cell motility and tumorigenesis, Sci. Rep (2017) 1–13. 10.1038/s41598-017-08225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Papierska K, Krajka-Kuźniak V, Paluszczak J, Kleszcz R, Skalski M, Studzińska-Sroka E, Baer-Dubowska W, Lichen-Derived Depsides and Depsidones Modulate the Nrf2, NF-κB and STAT3 Signaling Pathways in Colorectal Cancer Cells., Molecules 26 (2021). 10.3390/molecules26164787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Burlando B, Ranzato E, Volante A, Appendino G, Pollastro F, Verotta L, Antiproliferative effects on tumour cells and promotion of keratinocyte wound healing by different lichen compounds, Planta Med 75 (2009) 607–613. 10.1055/s-0029-1185329. [DOI] [PubMed] [Google Scholar]
- [34].Varol M, Natural Small-Molecules Obtained From Lichens as a Novel Source of Anti-Angiogenic Agents, J. Appl. Pharm 08 (2015) 1–2. 10.4172/1920-4159.1000e105. [DOI] [Google Scholar]
- [35].Hertog MG, Feskens EJ, Kromhout D, Hertog MG, Hollman PC, Hertog MG, Katan M, Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study, Lancet 342 (1993) 1007–1011. 10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- [36].Huxley RR, Neil HAW, The relation between dietary flavonol intake and coronary heart disease mortality: a meta-analysis of prospective cohort studies, Eur. J. Clin. Nutr 57 (2003) 904–908. 10.1038/sj.ejcn.1601624. [DOI] [PubMed] [Google Scholar]
- [37].Shukla V, Joshi GP, Rawat MSM, Lichens as a potential natural source of bioactive compounds: A review, Phytochem. Rev 9 (2010) 303–314. 10.1007/s11101-010-9189-6. [DOI] [Google Scholar]
- [38].Huneck S, The Significance of Lichens and Their Metabolites, Naturwissenschaften 86 (1999) 559–570. 10.1007/s001140050676. [DOI] [PubMed] [Google Scholar]
- [39].Nugraha AS, Laksono TA, Firli LN, Putri CPZS, Pratoko DK, Zulfikar Z, Untari LF, Wongso H, Lambert JM, Dillon CT, Keller PA, Anti-cancer Evaluation of Depsides Isolated from Indonesian Folious Lichens: Physcia millegrana, Parmelia dilatata and Parmelia aurulenta., Biomolecules 10 (2020). 10.3390/biom10101420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Letwin L, Malek L, Suntres Z, Christopher L, Cytotoxic and Antibiotic Potential of Secondary Metabolites from the Lichen Umbilicaria muhlenbergii., Curr. Pharm. Biotechnol 21 (2020) 1516–1527. 10.2174/1389201021666200504114515. [DOI] [PubMed] [Google Scholar]
- [41].Seo C, Choi YH, Ahn JS, Yim JH, Lee HK, Oh H, PTP1B inhibitory effects of tridepside and related metabolites isolated from the Antarctic lichen Umbilicaria antarctica, J. Enzyme Inhib. Med. Chem 24 (2009) 1133–1137. 10.1080/14756360802667811. [DOI] [PubMed] [Google Scholar]
- [42].Ouyang L, Shi Z, Zhao S, Wang F-T, Zhou T-T, Liu B, Bao J-K, Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis, Cell Prolif 45 (2012) 487–498. 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G, Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies., Aging (Albany. NY) 8 (2016) 603–619. 10.18632/aging.100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Balunas MJ, Kinghorn AD, Drug discovery from medicinal plants, Life Sci 78 (2005) 431–441. 10.1016/J.LFS.2005.09.012. [DOI] [PubMed] [Google Scholar]
- [45].Bačkorová M, Bačkor M, Mikeš J, Jendželovský R, Fedoročko P, Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid, Toxicol. Vitr 25 (2011) 37–44. 10.1016/j.tiv.2010.09.004. [DOI] [PubMed] [Google Scholar]
- [46].Lohezic-Le Devehat F, Legouin B, Couteau C, Boustie J, Coiffard L, Lichenic extracts and metabolites as UV filters, J. Photochem. Photobiol. B Biol 120 (2013) 17–28. 10.1016/j.jphotobiol.2013.01.009. [DOI] [PubMed] [Google Scholar]
- [47].Russo A, Piovano M, Lombardo L, Garbarino J, Cardile V, Lichen metabolites prevent UV light and nitric oxide-mediated plasmid DNA damage and induce apoptosis in human melanoma cells, Life Sci 83 (2008) 468–474. 10.1016/j.lfs.2008.07.012. [DOI] [PubMed] [Google Scholar]
- [48].Correcché ER, Enriz RD, Piovano M, Garbarino J, Gómez-Lechón MJ, Cytotoxic and apoptotic effects on hepatocytes of secondary metabolites obtained from lichens, ATLA Altern. to Lab. Anim 32 (2004) 605–615. 10.1177/026119290403200611. [DOI] [PubMed] [Google Scholar]
- [49].Varol M, Türk A, Candan M, Tay T, Koparal AT, Photoprotective Activity of Vulpinic and Gyrophoric Acids Toward Ultraviolet B-Induced Damage in Human Keratinocytes, Phyther. Res 30 (2016) 9–15. 10.1002/ptr.5493. [DOI] [PubMed] [Google Scholar]
- [50].Kumar KC S, Müller K, Lichen Metabolites. 2. Antiproliferative and Cytotoxic Activity of Gyrophoric, Usnic, and Diffractaic Acid on Human Keratinocyte Growth, J. Nat. Prod 62 (1999) 821–823. 10.1021/np980378z. [DOI] [PubMed] [Google Scholar]
- [51].Jeggo PA, Löbrich M, Contribution of DNA repair and cell cycle checkpoint arrest to the maintenance of genomic stability, DNA Repair (Amst) 5 (2006) 1192–1198. 10.1016/j.dnarep.2006.05.011. [DOI] [PubMed] [Google Scholar]
- [52].Ghate NB, Chaudhuri D, Sarkar R, Sajem AL, Panja S, Rout J, Mandal N, An antioxidant extract of tropical lichen, Parmotrema reticulatum, induces cell cycle arrest and apoptosis in breast carcinoma cell line MCF-7, PLoS One 8 (2013). 10.1371/journal.pone.0082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ren MR, Hur J-S, Kim JY, Park K-W, Park S-C, Seong C-N, Jeong I-Y, Byun M-W, Lee M-K, Seo K-I, Anti-proliferative effects of Lethariella zahlbruckneri extracts in human HT-29 human colon cancer cells., Food Chem. Toxicol 47 (2009) 2157–62. 10.1016/j.fct.2009.05.042. [DOI] [PubMed] [Google Scholar]
- [54].Su ZQ, Liu YH, Guo HZ, Sun CY, Xie JH, Li YC, Chen JN, Lai XP, Su ZR, Chen HM, Effect-enhancing and toxicity-reducing activity of usnic acid in ascitic tumor-bearing mice treated with bleomycin, Int. Immunopharmacol 46 (2017) 146–155. 10.1016/j.intimp.2017.03.004. [DOI] [PubMed] [Google Scholar]
- [55].Dinçsoy AB, Cansaran Duman D, Changes in apoptosis-related gene expression profiles in cancer cell lines exposed to usnic acid lichen secondary metabolite, Turkish J. Biol 41 (2017). 10.3906/biy-1609-40. [DOI] [Google Scholar]
- [56].Noh J-I, Mun S-K, Lim EH, Kim H, Chang D-J, Hur J-S, Yee S-T, Induction of Apoptosis in MDA-MB-231 Cells Treated with the Methanol Extract of Lichen Physconia hokkaidensis., J. Fungi (Basel, Switzerland) 7 (2021). 10.3390/jof7030188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yang Y, Bae WK, Nam SJ, Jeong MH, Zhou R, Park SY, Taş İ, Hwang YH, Park MS, Chung IJ, Kim KK, Hur JS, Kim H, Acetonic extracts of the endolichenic fungus EL002332 isolated from Endocarpon pusillum exhibits anticancer activity in human gastric cancer cells, Phytomedicine 40 (2018) 106–115. 10.1016/j.phymed.2018.01.006. [DOI] [PubMed] [Google Scholar]
- [58].Choudhary MI, Ali M, Wahab A, Khan A, Rasheed S, Shyaula SL, Rahman A, New antiglycation and enzyme inhibitors from Parmotrema cooperi, Sci. China Chem 54 (2011) 1926–1931. 10.1007/s11426-011-4436-2. [DOI] [Google Scholar]
- [59].Fournet M, Bonté F, Desmoulière A, Glycation Damage: A Possible Hub for Major Pathophysiological Disorders and Aging., Aging Dis 9 (2018) 880–900. 10.14336/AD.2017.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Thadhani VM, Karunaratne V, Potential of Lichen Compounds as Antidiabetic Agents with Antioxidative Properties: A Review, Oxid. Med. Cell. Longev 2017 (2017). 10.1155/2017/2079697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Huo X, Qiao L, Chen Y, Chen X, He Y, Zhang Y, Discovery of Novel Multi-target Inhibitor of angiotensin type 1 receptor and neprilysin inhibitors from Traditional Chinese Medicine, Sci. Rep 9 (2019) 1–10. 10.1038/s41598-019-52309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yousuf S, Choudhary MI, Atta-Ur-Rahman, Lichens: Chemistry and biological activities, in: Stud. Nat. Prod. Chem, Elsevier B.V., 2014: pp. 223–259. 10.1016/B978-0-444-63430-6.00007-2. [DOI] [Google Scholar]
- [63].Kenner KA, Anyanwu E, Olefsky JM, Kusari J, Protein-tyrosine Phosphatase 1B Is a Negative Regulator of Insulin- and Insulin-like Growth Factor-I-stimulated Signaling*, J. Biol. Chem 271 (1996) 19810–19816. 10.1074/jbc.271.33.19810. [DOI] [PubMed] [Google Scholar]
- [64].Bui V-M, Duong T-H, Chavasiri W, Nguyen K-P-P, Huynh B-L-C, A new depsidone from the lichen Usnea ceratina Arch, Nat. Prod. Res (2020) 1–7. 10.1080/14786419.2020.1828405. [DOI] [PubMed] [Google Scholar]
- [65].Duong T-H, Hang T-X-H, Le Pogam P, Tran T-N, Mac D-H, Dinh M-H, Sichaem J, α-Glucosidase Inhibitory Depsidones from the Lichen Parmotrema tsavoense., Planta Med 86 (2020) 776–781. 10.1055/a-1179-1050. [DOI] [PubMed] [Google Scholar]
- [66].Chae H-J, Kim G-J, Deshar B, Kim H-J, Shin M-J, Kwon H, Youn U-J, Nam J-W, Kim S-H, Choi H, Suh S-S, Anticancer Activity of 2-O-caffeoyl Alphitolic Acid Extracted from the Lichen, Usnea barbata 2017-KL-10., Molecules 26 (2021). 10.3390/molecules26133937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhang Y, Tan CY, Spjut RW, Fuchs JR, Kinghorn AD, Rakotondraibe LH, Specialized metabolites of the United States lichen Niebla homalea and their antiproliferative activities, Phytochemistry 180 (2020) 112521. 10.1016/j.phytochem.2020.112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Singh N, Nambiar D, Kale RK, Singh RP, Usnic acid inhibits growth and induces cell cycle arrest and apoptosis in human lung carcinoma A549 cells., Nutr. Cancer 65 Suppl 1 (2013) 36–43. 10.1080/01635581.2013.785007. [DOI] [PubMed] [Google Scholar]
- [69].Qi W, Lu C, Huang H, Zhang W, Song S, Liu B, (+)-Usnic Acid Induces ROS-dependent Apoptosis via Inhibition of Mitochondria Respiratory Chain Complexes and Nrf2 Expression in Lung Squamous Cell Carcinoma., Int. J. Mol. Sci 21 (2020). 10.3390/ijms21030876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wu W, Hou B, Tang C, Liu F, Yang J, Pan T, Si K, Lu D, Wang X, Wang J, Xiong X, Liu J, Xie C, (+)-Usnic Acid Inhibits Migration of c-KIT Positive Cells in Human Colorectal Cancer, Evidence-Based Complement. Altern. Med 2018 (2018) 5149436. 10.1155/2018/5149436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Değerli E, Torun V, Cansaran-Duman D, miR-185–5p response to usnic acid suppresses proliferation and regulating apoptosis in breast cancer cell by targeting Bcl2., Biol. Res 53 (2020) 19. 10.1186/s40659-020-00285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kılıç N, Değerli E, Torun V, Altaytaş F, Investigation of Synergistic effect of Tamoxifen and Usnic Acid on Breast Cancer Cell Line, 1 (2016) 2–4. [Google Scholar]
- [73].Sun TX, Li MY, Zhang ZH, Wang JY, Xing Y, Ri M, Jin CH, Xu GH, Piao LX, Jin HL, Zuo HX, Ma J, Jin X, Usnic acid suppresses cervical cancer cell proliferation by inhibiting PD-L1 expression and enhancing T-lymphocyte tumor-killing activity, Phyther. Res 35 (2021) 3916–3935. 10.1002/ptr.7103. [DOI] [PubMed] [Google Scholar]
- [74].Yang Y, Bhosle SR, Yu YH, Park S-Y, Zhou R, Taş İ, Gamage CDB, Kim KK, Pereira I, Hur J-S, Ha H-H, Kim H, Tumidulin, a Lichen Secondary Metabolite, Decreases the Stemness Potential of Colorectal Cancer Cells, Molecules 23 (2018) 2968. 10.3390/molecules23112968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Petrova K, Kello M, Kuruc T, Backorova M, Petrovova E, Vilkova M, Goga M, Rucova D, Backor M, Mojzis J, Potential Effect of Pseudevernia furfuracea (L.) Zopf Extract and Metabolite Physodic Acid on Tumour Microenvironment Modulation in MCF-10A Cells., Biomolecules 11 (2021). 10.3390/biom11030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sanjaya A, Avidlyandi A, Adfa M, Ninomiya M, Koketsu M, A New Depsidone from Teloschistes flavicans and the Antileukemic Activity, J. Oleo Sci 69 (2020) 1591–1595. 10.5650/jos.ess20209. [DOI] [PubMed] [Google Scholar]
- [77].Huynh BLC, Bui VM, Nguyen KPP, Pham NKT, Nguyen TP, Three new diphenyl ethers from the lichen Parmotrema praesorediosum (Nyl.) Hale (Parmeliaceae), Nat. Prod. Res (2020) 1–7. 10.1080/14786419.2020.1837818. [DOI] [PubMed] [Google Scholar]
- [78].Kilic N, Aras S, Cansaran-Duman D, Determination of Vulpinic Acid Effect on Apoptosis and mRNA Expression Levels in Breast Cancer Cell Lines, Anticancer. Agents Med. Chem 18 (2018). 10.2174/1871520618666180903101803. [DOI] [PubMed] [Google Scholar]
- [79].Solár P, Hrčková G, Koptašíková L, Velebný S, Solárová Z, Bačkor M, Murine breast carcinoma 4T1 cells are more sensitive to atranorin than normal epithelial NMuMG cells in vitro: Anticancer and hepatoprotective effects of atranorin in vivo, Chem. Biol. Interact 250 (2016) 27–37. 10.1016/J.CBI.2016.03.012. [DOI] [PubMed] [Google Scholar]
- [80].Suh SS, Kim TK, Kim JE, Hong JM, Nguyen TTT, Han SJ, Youn UJ, Yim JH, Kim IC, Anticancer activity of ramalin, a secondary metabolite from the antarctic lichen ramalina terebrata, against colorectal cancer cells, Molecules 22 (2017). 10.3390/molecules22081361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Yang Y, Park S-Y, Nguyen TT, Yu YH, Van Nguyen T, Sun EG, Udeni J, Jeong M-H, Pereira I, Moon C, Ha H-H, Kim KK, Hur J-S, Kim H, Lichen Secondary Metabolite, Physciosporin, Inhibits Lung Cancer Cell Motility, PLoS One 10 (2015) e0137889–e0137889. 10.1371/journal.pone.0137889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Nguyen TT, Yoon S, Yang Y, Bin Lee H, Oh S, Jeong MH, Kim JJ, Yee ST, Crişan F, Moon C, Lee KY, Kim KK, Hur JS, Kim H, Lichen secondary metabolites in Flavocetraria cucullata Exhibit anti-cancer effects on human cancer cells through the induction of apoptosis and suppression of tumorigenic potentials, PLoS One 9 (2014). 10.1371/journal.pone.0111575. [DOI] [PMC free article] [PubMed] [Google Scholar]


