Abstract
Background
Osteochondral allograft (OCA) transplantation is an increasingly common treatment for patients with symptomatic focal chondral lesions of the knee. There has been increasing interest in determining predictive factors to maximize patient benefit after this operation. The aim of the present study is to evaluate the predictive association of the physical component (PCS) and mental component (MCS) scores of the Short Form 36 (SF-36) questionnaire for achievement of the minimal clinically important difference (MCID) after OCA transplantation.
Methods
This retrospective study of a longitudinally maintained institutional registry included 91 patients who had undergone OCA transplantation for symptomatic focal osteochondral lesions of the femoral condyle. Included patients were those with complete preoperative questionnaires for the SF-36 and IKDC and completed postoperative IKDC at 2-year follow-up. Multivariate analysis was performed evaluating predictive association of the preoperative MCS and PCS with achievement of the MCID for the IKDC questionnaire.
Results
Logistic multivariate modeling demonstrated a statistically significant association between lower preoperative PCS and achievement of the MCID (P = 0.022). A defect diameter >2 cm was also associated with achievement of MCID (P = 0.049). Preoperative MCS did not demonstrate a significant association (P = 0.09) with achievement of the MCID.
Conclusions
For this cohort of 91 patients, the preoperative SF-36 PCS and lesion size were predictive of achievement of the MCID at 2-year follow-up after femoral OCA transplantation. These findings support an important role of baseline physical health scores for predicting which patients will obtain a meaningful clinical benefit from this surgery.
Keywords: cartilage transplantation, grafts, outcome measures, general, articular cartilage, tissue
Introduction
Osteochondral allograft (OCA) transplantation is an increasingly common treatment for patients with symptomatic osteochondral lesion of the knee articular surface. Short and midterm outcomes for this procedure demonstrate promising results with regard to pain relief, patient-reported outcomes (PROs), and return to sport.1,2 The minimally clinically important difference (MCID) for this procedure has been calculated for several PROs,3,4 thereby providing a foundation for further investigations into predictive factors for outcomes following this procedure.
The patient-specific factors that predict improvement after OCA surgery remains a subject of ongoing study.3,5 Previous investigations have evaluated the potential role of a number of patient-specific factors in predicting achievement of the MCID. 3 One potentially important factor is preoperative Short Form-36 (SF-36) score. The SF-36 questionnaire was developed as a marker for patient general health.6,7 This questionnaire has been validated for a number of orthopedic conditions and surgeries. 7 Notably, based on the 8 categories of patient experience, separate component scores related to mental health (mental health component score or MCS) and physical health (physical health component score or PCS) may be calculated from SF-36 questionnaires to allow more specific evaluation of a patient’s condition. 7
With regard to the SF-36 component scores, it has been previously determined that the MCS does not adequately predict outcomes after OCA 8 ; however, given the overlap between the categories weighed in the MCS and PCS, it would seem to be important to evaluate the effect of both of these components on achievement of clinical outcomes. Therefore, the purpose of the present study is to determine if the MCS or PCS are independent predictive variables for achievement of the MCID for the International Knee Documentation Committee questionnaire (IKDC) after OCA for femoral condyle lesions. We hypothesized that the MCS and PCS would be predictive of achievement of the MCID for the IKDC.
Materials and Methods
Study Design
A retrospective query of a prospectively collected institutional registry was performed for patients undergoing OCA from 1999 to 2017. The institutional registry is approved by an institutional review board (IRB 2013-024). Informed consent was obtained for all patients included in the registry.
Participants
Inclusion criteria for this study were (1) symptomatic full-thickness lesion of the femoral cartilage (Outerbridge grade IV), (2) treatment with fresh OCA, (3) completion of a baseline SF-36 questionnaire, and (4) completion of the IKDC questionnaire preoperatively and at 2-year follow-up. Patients who underwent concomitant anterior cruciate ligament (ACL) reconstruction were included in the analysis.
Exclusion criteria were individuals with incomplete pre- or postoperative data, treatment of tibial or patellar chondral lesions with osteochondral graft, concurrent realignment surgery (i.e., osteotomy), or treatment with a cartilage restoration procedure aside from OCA (e.g., particulated juvenile cartilage, autologous chondrocyte implantation, microfracture).
Study Population Selection and Data Collection
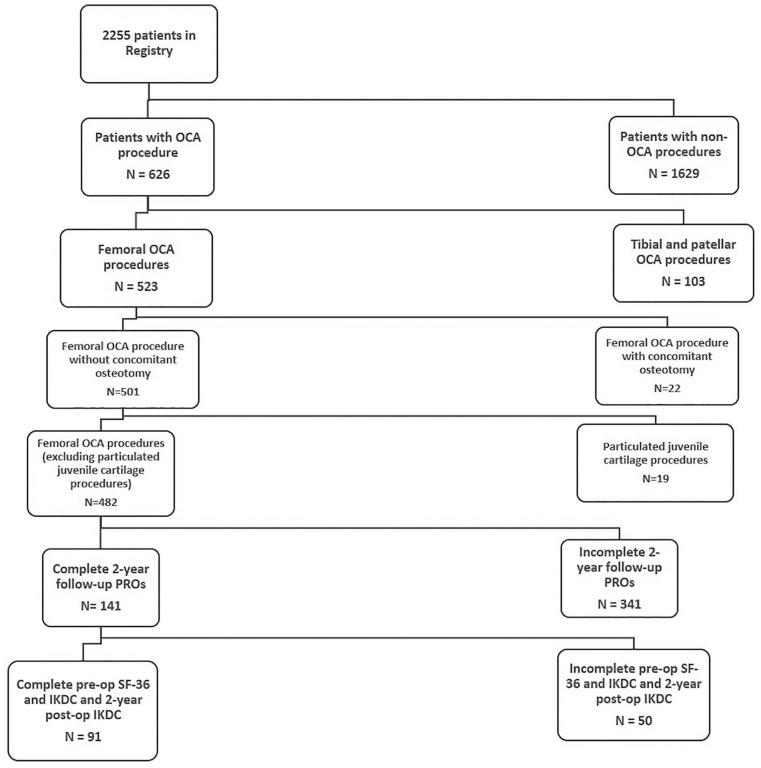
Of the total 2255 patients included in the registry, a total of 141 individuals were identified having undergone allograft transplantation for treatment of femoral osteochondral lesions with 2-year follow-up PROs. Of that sample, 91 patients were identified who had completed the preoperative SF-36 and IKDC and 2-year postoperative IKDC (see Fig. 1 ).
Figure 1.
Diagram of patient cohort. OCA = osteochondral allograft; PRO = patient-reported outcome; IKDC = International Knee Documentation Committee Questionnaire; SF-36 = Short Form-36.
In addition to baseline SF-36 and IKDC questionnaire scores, baseline demographic data including age, sex, and body mass index were collected. Additionally, the number and type of previous ipsilateral knee surgical procedures was collected.
Indications and Surgical Technique
OCA was selected as the treatment option for patients based on clinical judgement with regard to defect size and depth, location, and patient functional goals. Generally, OCA was selected for treatment of osteochondral lesions with diameter greater than 2 cm.
All surgical procedures were performed by fellowship-trained orthopedic surgeons at a single institution with extensive experience in cartilage restoration procedures. Following examination under anesthesia, an initial diagnostic arthroscopy was performed to confirm the size and location of the chondral lesion and address co-existing pathology as indicated (i.e., chondroplasty, meniscal surgery, or ACL reconstruction). After completion of the arthroscopic portion of the procedure, either a medial or lateral parapatellar arthrotomy was performed to allow optimal visualization of the lesion. The defect was then debrided to stable cartilage margins.
Fresh OCA implantation was then performed in accordance with the previously described dowel technique. 9 Fresh, cold-stored OCAs were obtained from commercially available sources. All donor tissue was screened and processed according to the standards of the American Association of Tissue Banks. Grafts were implanted on average 28.15 days after harvest.
Once the chondral defect was adequately visualized, the lesion was sized and reamed to a bed of healthy bone. A dowel of identical size was then harvested from the donor condyle. Lesion depth was carefully measured and matched to the donor tissue. The harvested grafts were then impacted into the lesion site, maintaining press-fit fixation.
With regard to postoperative rehabilitation protocol, patients remained touchdown or non–weight bearing for 1 to 2 weeks after surgery, with gradual progression to full weight-bearing between weeks 4 and 6. Full range of motion was permitted immediately and encouraged. Brace wear was discontinued at 3 to 6 weeks based on restoration of adequate quadriceps function. A supervised physical therapy protocol was undertaken in all cases to aid in the restoration of normal gait, muscle strength, endurance, balance, and proprioception. Timeline to return to participation in desired activities was determined on an individual basis based on the patient’s level of activity and achievement of functional milestones as they progressed through the postoperative rehabilitation program.
Assessment of Outcomes
General health outcomes for each patient was assessed with the SF-36 v 1.0. 6 Based on the method described by Laucis et al., the MCS and PCS were calculated based on a weighted scoring system of the 8 domains of the SF-36 questionnaire. 7
Preoperative and 2-year follow-up scores for the IKDC questionnaire were collected. The IKDC is a knee-specific measure of function and symptoms that is validated for treatment of cartilage lesions.10,11 The MCID for osteochondral grafting has been previously established for the IKDC via the anchor method. 3
Statistical Analysis
Descriptive statistics were used to report baseline and postoperative characteristics. Continuous variables included age at the time of surgery, body mass index (BMI), preoperative MCS, and preoperative PCS. Binary variables included gender, previous surgery, concomitant ACL reconstruction, and defect diameter >2 cm.
An initial univariate test was performed for each covariate against the primary outcome (achievement of an improvement of 17 points on the IKDC at 2 years after surgery). Continuous variables were evaluated using a Wilcoxon rank-sum test. Binary variables were evaluated using a chi-squared test.
Multivariable logistic regression models were fitted to investigate the predictors of achieving IKDC MCID by 2 years after OCA transplantation (improvement of 17 points). 3 All preoperative covariates were included in the initial model. Variables that fell out of the model due to lack of significance are not presented in the tables. Statistically significant association with the primary outcome was defined as P < 0.05 in the multivariate model. All analyses were performed using SAS Software version 9.4 (SAS Institute, Cary, NC).
Results
Patient Demographics and Characteristics
Of the initial cohort of 141 patients, 50 patients were excluded for incomplete baseline (SF-36, IKDC) or 2-year postoperative (IKDC) questionnaires. Mean follow-up was 26.13 months (range 23.54-32.38). The mean age for the final cohort of 91 patients was 42.6 years (standard deviation [SD] 12.96). With regard to gender, the cohort was 63% (n = 57) male. The mean BMI was 25.9 (SD 3.91). A total of 68.2% (n = 62) of patients had undergone previous surgery; 11.4% (n = 10) of the patients had concomitant ACL reconstruction; and 68.1% (n = 61) of patients had a defect diameter greater than or equal to 2 cm. Preoperative MCS was 50.07 (SD 9.39), while preoperative PCS was 44.10 (SD 8.53). A sensitivity analysis conducted to detect differences between the cohorts with complete and incomplete 2-year follow-up demonstrated no significant difference in any of the above variables ( Table 1 ).
Table 1.
Sensitivity Analysis Comparing Baseline Demographics between Cohorts with Complete and Missing Data.
| Demographics | Complete Data (91) | Missing Data (50) | P Value |
|---|---|---|---|
| Age | 42.57 (12.96) | 39 (12.16) | 0.11 |
| Gender | Male 57 (62.64%) | Male 38 (76%) | 0.13 |
| BMI | 25.88 (3.91) | 25.54 (4.17) | 0.38 |
| Previous surgery | Yes 60 (68.18) | Yes 33 (70.21%) | 0.85 |
| Concomitant ACL | Yes 10 (11.36) | Yes 4 (8.7%) | 0.77 |
| Defect diameter | <2 cm (29, 31.87%) | <2 cm (13, 26%) | 0.56 |
| Preoperative MCS | 50.07 (9.39) | 49.31 (9.25) | 0.43 |
| Preoperative PCS | 44.10 (8.53) | 43.49 (9.46) | 0.81 |
BMI = body mass index; ACL = anterior cruciate ligament; MCS = mental health component score; PCS = physical health component score.
The mean preoperative IKDC score was 47.8 (SD 15.79, range 13.8-81.6) in the final cohort. No ceiling or floor effect was detected for the IKDC in this cohort (defined as ≥15% of the study population in the top or bottom 5%). At 2 years after surgery the mean IKDC score was 70.3 (SD 19.91). The average change in IKDC over this 2-year period was 24.6 (SD 16.53). Using the previously published IKDC MCID of 17, 3 60% of the cohort met MCID.
Logistic multivariate modeling demonstrated a statistically significant association between lower preoperative PCS and achievement of the MCID (P = 0.022). A defect diameter >2 cm was also associated with achievement of MCID (P = 0.049). There was a trend toward a negative association with age (P = 0.07), and a positive association between preoperative MCS (P = 0.09) and achievement of the MCID that did not achieve the threshold for statistical significance. The complete findings of the univariate and multivariate analysis are detailed in Tables 2 and 3 .
Table 2.
Univariate Analysis.
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Age | 0.98 (0.94-1.01) | 0.14 |
| Female vs. male | 0.64 (0.26-1.56) | 0.33 |
| BMI | 1.04 (0.93-1.164) | 0.48 |
| Previous surgery | 0.81 (0.32-2.06) | 0.66 |
| ACL | 0.60 (0.16-2.27) | 0.45 |
| Defect size | 2.52 (1.02-6.24) | 0.04 |
| Preoperative MCS | 1.01 (0.97-1.06) | 0.63 |
| Preoperative PCS | 0.96 (0.91-1.01) | 0.12 |
CI = confidence interval; BMI = body mass index; ACL = anterior cruciate ligament; MCS = mental health component score; PCS = physical health component score.
Table 3.
Multivariate Analysis.
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Age | 0.97 (0.93-1.00) | 0.07 |
| Size | 2.64 (1.00-6.97) | 0.0492 |
| Preoperative MCS | 1.06 (0.99-1.13) | 0.09 |
| Preoperative PCS | 0.92 (0.85-0.99) | 0.02 |
CI = confidence interval; MCS = mental health component score; PCS = physical health component score.
Discussion
The present study demonstrated that lower preoperative PCS and defect size >2 cm are positive predictors for achievement of the MCID for this cohort of patients undergoing femoral OCA transplantation. Notably, the preoperative MCS was not a statistically significant predictor for patient outcome. In setting of increasing frequency of OCA transplantation, these findings have important implications with regard to the role of patient perception of preoperative physical and mental health.
There has been increasing focus on the role of a patient’s perception of baseline health as it relates to outcomes after orthopedic procedures.12-15 This is of particular interest in the field of sports medicine, given the tendency for higher functional expectations in this patient population. To this end, the preoperative physical and mental component summaries of the SF-36 offer valuable insights into a patient’s baseline perception of mental and physical health. Although several investigations have evaluated the predictive role of baseline patient factors in cartilage restoration surgery,3,8,21 there are little data evaluating the role of both SF-36 composite scores in this setting. As such, our study is unique in the inclusion of both the PCS and MCS in our predictive model.
We believe that a compelling finding of our investigation is the negative predictive association of the PCS for achievement of the MCID. This observation suggests that patients with a higher level of preoperative physical fitness may have less opportunity to achieve a clinically meaningful degree of improvement after surgery. Similar observations in patients with higher baseline PROs have previously been reported throughout the orthopedic literature.16-19 In congruence with our findings, Wang et al. demonstrated that patients with higher baseline IKDC and Knee Outcome Survey-Activities of Daily Living (KOS-ADL) were less likely to achieve the MCID for OCA and autograft transplantation. 3 These findings may assist clinicians in preoperative discussions with patients who are considering cartilage restoration with OCA transplantation.
For the present study, preoperative MCS did not have a significant association with achievement of the MCID (P = 0.09). The lack of statistical significance may be a function of the relatively small sample size in the present study; however, previous investigators have demonstrated similar findings. Specifically, Ackermann et al. 8 found that the MCS had no predictive association with PRO measures in a sample of 67 patients undergoing OCA transplantation. While the Ackermann study did not specifically evaluate achievement of the MCID, their findings and our own call into question the ability of preoperative MCS to predict outcomes for this surgery. It should be noted that MCS is not a perfect proxy for mental health. In fact, previous investigations have questioned the relationship between the MCS and formal evaluation of mental health. 20 As such, prospective investigation with a larger cohort and more rigorous evaluation of baseline mental health is merited as it relates to management of cartilage lesions in active patients.
The relationship between defect size and cartilage restoration treatment outcomes remains controversial. This is particularly true for smaller lesions, for which treatment options such as microfracture and OATS may be recommended.21,22 Nevertheless, OCA transplantation remains a well-established option for lesions of all sizes and should be considered on a case by case basis depending on patient profile.23,24 For this study cohort, patients with lesion size >2 cm were more likely to obtain the MCID (P = 0.049) at 2-year follow-up. These findings may be the result of patients with larger, more symptomatic lesions having more room to benefit from surgery. These findings are controversial with regard to the existing literature. Previously, Tirico et al. 25 noted that there was no size-based difference in survivorship or PROs at 5 and 10 years postoperation in their cohort of 156 knees. Notably, the authors did note a larger change in IKDC from baseline to final follow-up for larger allografts. This specific finding may explain the contrasting findings in our own study.
Our findings did not demonstrate a statistically significant relationship between age at the time of surgery and achievement of the MCID (P = 0.07). Previous investigators have noted a relationship between age at the time of surgery and outcomes of cartilage restoration surgery.5,26-30 Specific to OCA transplantation, Frank et al. reported conflicting data compared to the present study. 5 In a cohort of 170 patients, the authors found that patients younger than 40 years old demonstrated a lower mean KOOS (Knee Injury and Osteoarthritis Outcome Score) score than patients older than 40. Frank et al. suggest that this observation may indicate a higher level of functional expectation for younger patients undergoing this procedure. Differences in study methodology may explain the different result shown in our study. Notably, our primary outcome was achievement of the MCID, which speaks to the clinical benefit observed by the patient after surgery. Further study into the relationship between age and OCA transplantation outcome is merited given these conflicting findings.
Limitations
There are several limitations that should be considered in interpretation of these results. The sample size for the cohort is relatively small and was further limited by incomplete preoperative and postoperative PRO questionnaires, which may introduce an element of selection bias. For this reason, we performed a sensitivity analysis comparing the baseline characteristics of those patients missing postoperative data with our study group, demonstrating no significant difference (see Table 1 ). As such, we are relatively confident that our findings would remain unchanged if data for all 141 patients were available, although the effect of patient satisfaction on their desire to follow-up is a potential source of bias. We also acknowledge that these results are limited by the short-term follow-up for the included patients. Further study with larger cohort sizes and longer follow-up is merited to further explore the relationships suggested by our findings.
Caution should be taken prior to generalizing these results to all patients undergoing cartilage restoration. While physical component summary score proved a significant predictor of surgical outcome for this cohort, other factors may play a role for different patient populations. The findings of the present investigation are valuable in that they establish SF-36 component scores as important factors to consider in predictive modeling for cartilage restoration surgery outcomes. There is increasing interest in the application of predictive modeling techniques to large registry databases in orthopedics. This interest has expanded to include the utilization of advanced statistical techniques, such as machine learning.31-34 As these modalities continue to increase in popularity, it will be important to establish the key baseline/preoperative variables to include in predictive modeling. The present study establishes the PCS of the SF-36 as one such variable and suggest that further detailed investigation of the role of preoperative mental health on outcomes after OCA transplantation is merited.
Conclusion
For this cohort of 91 patients, the preoperative SF-36 PCS and lesion size were predictive of achievement of the MCID at 2-year follow-up after femoral OCA transplantation. These findings support an important role of baseline physical health scores for predicting which patients will obtain a meaningful clinical benefit from this surgery.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Rodeo: Consultant: Advance Medical; Stock options: Ortho RTI; Research support: NIH OREF Virginia Toulmin Foundation, Tisch Family Foundation, AOSSM JRF; Ortho Editorial Board: American Journal of Sports Medicine. Owusu-Akyaw: Editorial Board: Arthroscopy Board of Directors. J Robert Gladden Orthopaedic Society. The other authors have no conflicts of interest to disclose.
Ethical Approval: A retrospective query of a prospectively collected institutional registry was performed for patients undergoing OCA from 1999 to 2017. The institutional registry is approved by an institutional review board (IRB 2013-024).
Informed Consent: Informed consent was obtained for all patients included in the registry.
ORCID iD: Kwadwo A. Owusu-Akyaw  https://orcid.org/0000-0002-3661-8844
https://orcid.org/0000-0002-3661-8844
References
- 1. Frank RM, Lee S, Levy D, Poland S, Smith M, Scalise N, et al. Osteochondral allograft transplantation of the knee: analysis of failures at 5 years. Am J Sports Med. 2017;45(4):864-74. [DOI] [PubMed] [Google Scholar]
- 2. Levy YD, Gortz S, Pulido PA, McCauley JC, Bugbee WD. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res. 2013;471(1_suppl):231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang D, Chang B, Coxe FR, Pais MD, Wickiewicz TL, Warren RF, et al. Clinically meaningful improvement after treatment of cartilage defects of the knee with osteochondral grafts. Am J Sports Med. 2019;47(1_suppl):71-81. [DOI] [PubMed] [Google Scholar]
- 4. Ogura T, Ackermann J, Mestriner AB, Merkely G, Gomoll AH. The minimal clinically important difference and substantial clinical benefit in the patient-reported outcome measures of patients undergoing osteochondral allograft transplantation in the knee. Cartilage. 2018:1947603518812552. doi: 10.1177/1947603518812552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frank RM, Cotter EJ, Lee S, Poland S, Cole BJ. Do outcomes of osteochondral allograft transplantation differ based on age and sex? A comparative matched group analysis. Am J Sports Med. 2018;46(1_suppl):181-91. [DOI] [PubMed] [Google Scholar]
- 6. Patel AA, Donegan D, Albert T. The 36-item short form. J Am Acad Orthop Surg. 2007;15:126-34. [DOI] [PubMed] [Google Scholar]
- 7. Laucis NC, Hays RD, Bhattacharyya T. Scoring the SF-36 in orthopaedics: a brief guide. J Bone Joint Surg Am. 2015;97(19):1628-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ackermann J, Ogura T, Duerr RA, Mestriner AB, Gomoll AH. Mental health has no predictive association with self-assessed knee outcome scores in patients after osteochondral allograft transplantation of the knee. Orthop J Sports Med. 2018;6(12):2325967118812363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams RJ, 3rd, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89(4):718-26. [DOI] [PubMed] [Google Scholar]
- 10. Hambly K, Griva K. IKDC or KOOS? Which measures symptoms and disabilities most important to postoperative articular cartilage repair patients? Am J Sports Med. 2008;36(9):1695-704. [DOI] [PubMed] [Google Scholar]
- 11. Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600-13. [DOI] [PubMed] [Google Scholar]
- 12. Mayo BC, Massel DH, Bohl DD, Narain AS, Hijji FY, Long WW, et al. Preoperative mental health status may not be predictive of improvements in patient-reported outcomes following an anterior cervical discectomy and fusion. J Neurosurg Spine. 2017;26(2):177-82. [DOI] [PubMed] [Google Scholar]
- 13. Lai SWH, Tang CQY, Graetz AEK, Thevendran G. Preoperative mental health score and postoperative outcome after hallux valgus surgery. Foot Ankle Int. 2018;39(12):1403-9. [DOI] [PubMed] [Google Scholar]
- 14. Nwachukwu BU, Adjei J, Rauck RC, Chahla J, Okoroha KR, Verma NN, et al. How much do psychological factors affect lack of return to play after anterior cruciate ligament reconstruction? A systematic review. Orthop J Sports Med. 2019;7(5):2325967119845313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nwachukwu BU, Voleti PB, Berkanish P, Chang B, Cohn MR. Return to play and patient satisfaction after ACL reconstruction: study with minimum 2-year follow-up. J Bone Joint Surg Am. 2017;99(9):720-5. [DOI] [PubMed] [Google Scholar]
- 16. Berliner JL, Brodke DJ, Chan V, SooHoo NF, Bozic KJ. John Charnley Award: preoperative patient-reported outcome measures predict clinically meaningful improvement in function after THA. Clin Orthop Relat Res. 2016;474(2):321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berliner JL, Brodke DJ, Chan V, SooHoo NF, Bozic KJ. Can preoperative patient-reported outcome measures be used to predict meaningful improvement in function after TKA? Clin Orthop Relat Res. 2017;475(1_suppl):149-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nwachukwu BU, Fields K, Chang B, Nawabi DH, Kelly BT, Ranawat AS. Preoperative outcome scores are predictive of achieving the minimal clinically important difference after arthroscopic treatment of femoroacetabular impingement. Am J Sports Med. 2017;45(3):612-9. [DOI] [PubMed] [Google Scholar]
- 19. Werner BC, Chang B, Nguyen JT, Dines DM, Gulotta LV. What change in American shoulder and elbow surgeons score represents a clinically important change after shoulder arthroplasty? Clin Orthop Relat Res. 2016;474(12):2672-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhandari M, Busse JW, Hanson BP, Leece P, Ayeni OR, Schemitsch EH. Psychological distress and quality of life after orthopedic trauma: an observational study. Can J Surg. 2008;51(1_suppl):15-22. [PMC free article] [PubMed] [Google Scholar]
- 21. Gomoll AH, Farr J, Gillogly SD, Kercher JS, Minas T. Surgical management of articular cartilage defects of the knee. Instr Course Lect. 2011;60:461-83. [PubMed] [Google Scholar]
- 22. Bekkers JE, Inklaar M, Saris DB. Treatment selection in articular cartilage lesions of the knee: a systematic review. Am J Sports Med. 2009;37(Suppl 1):148S-155S. [DOI] [PubMed] [Google Scholar]
- 23. Torrie AM, Kesler WW, Elkin J, Gallo RA. Osteochondral allograft. Curr Rev Musculoskelet Med. 2015;8(4):413-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pisanu G, Cottino U, Rosso F, Blonna D, Marmotti AG, Bertolo C, et al. Large osteochondral allografts of the knee: surgical technique and indications. Joints. 2018;6(1_suppl):42-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tirico LEP, McCauley JC, Pulido PA, Bugbee WD. Lesion size does not predict outcomes in fresh osteochondral allograft transplantation. Am J Sports Med. 2018;46(4):900-7. [DOI] [PubMed] [Google Scholar]
- 26. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):1986-96. [DOI] [PubMed] [Google Scholar]
- 27. Gudas R, Gudaite A, Mickevicius T, Masiulis N, Simonaityte R, Cekanauskas E, et al. Comparison of osteochondral autologous transplantation, microfracture, or debridement techniques in articular cartilage lesions associated with anterior cruciate ligament injury: a prospective study with a 3-year follow-up. Arthroscopy. 2013;29(1_suppl):89-97. [DOI] [PubMed] [Google Scholar]
- 28. Gudas R, Gudaite A, Pocius A, Gudiene A, Cekanauskas E, Monastyreckiene E, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-508. [DOI] [PubMed] [Google Scholar]
- 29. Krishnan SP, Skinner JA, Bartlett W, Carrington RW, Flanagan AM, Briggs TW, et al. Who is the ideal candidate for autologous chondrocyte implantation? J Bone Joint Surg Br. 2006;88(1_suppl):61-94. [DOI] [PubMed] [Google Scholar]
- 30. Nawaz SZ, Bentley G, Briggs TW, Carrington RW, Skinner JA, Gallagher KR, et al. Autologous chondrocyte implantation in the knee: mid-term to long-term results. J Bone Joint Surg Am. 2014;96(10):824-30. [DOI] [PubMed] [Google Scholar]
- 31. Fontana MA, Lyman S, Sarker GK, Padgett DE, MacLean CH. Can machine learning algorithms predict which patients will achieve minimally clinically important differences from total joint arthroplasty? Clin Orthop Relat Res. 2019;477(6):1267-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bayliss L, Jones LD. The role of artificial intelligence and machine learning in predicting orthopaedic outcomes. Bone Joint J. 2019;101-B(12):1476-8. [DOI] [PubMed] [Google Scholar]
- 33. Du Y, Almajalid R, Shan J, Zhang M. A Novel method to predict knee osteoarthritis progression on MRI using machine learning methods. IEEE Trans Nanobioscience. 2018;17(3):228-36. [DOI] [PubMed] [Google Scholar]
- 34. Navarro SM, Wang EY, Haeberle HS, Mont MA, Krebs VE, Patterson BM, et al. Machine learning and primary total knee arthroplasty: patient forecasting for a patient-specific payment model. J Arthroplasty. 2018;33(12):3617-23. [DOI] [PubMed] [Google Scholar]