Abstract
Objective
To determine the role of magnetic resonance imaging (MRI) MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) 1 and 2.0 scores in the assessment of postoperative outcome after autologous matrix-induced chondrogenesis (AMIC) for the treatment of osteochondral lesions of the talus (OLTs). It was hypothesized that preoperative patient factors or OLT morphology are associated with postoperative MOCART scores; yet postoperative clinical outcome is not.
Study Design
Cohort study; Level of evidence, 4. This study evaluated isolated AMIC that were implanted on the talus of 35 patients for the treatment of symptomatic OLT. Tegner and AOFAS (American Orthopaedic Foot and Ankle Society) scores were obtained at an average follow-up of 4.5 ± 1.8 years and postoperative MRI scored according to the MOCART 1 and 2.0.
Results
OLT size showed significant correlation with postoperative MRI scores (MOCART 1: P = 0.006; MOCART 2.0: P = 0.004). Bone grafting was significantly associated with a MOCART 1 subscale (signal intensity of repair tissue; P = 0.038). Age and defect size showed significant correlations with MOCART 2.0 subscales (P < 0.05). Patients with shorter follow-up had a significantly higher MOCART 1 score and a trend toward better MOCART 2.0 scores than patients with longer follow-up (64.7 vs. 52.9 months, P = 0.02; 69.4 vs. 60.6 months, P = 0.058). No MOCART score was associated with postoperative patient-reported outcomes (n.s.).
Conclusion
Osteochondral lesion size is associated with postoperative MOCART scores in patients treated with AMIC for OLTs, with decreasing MOCART scores over time. Yet clinical outcome does not correlate with any MOCART score. Thus, MOCART assessment seems to have no significant role in the postoperative treatment of asymptomatic patients that underwent AMIC for OLTs.
Keywords: autologous matrix-induced chondrogenesis, osteochondral lesion, talus, MOCART, osteoarthritis, cartilage repair, AMIC
Introduction
Osteochondral lesions of the talus (OLTs are defined as damage to the coating cartilage and subchondral bone due to repetitive microtrauma or traumatic injury to the ankle joint1-3 whether through direct trauma (i.e., sports)4-6 or joint instability.7-9 Native hyaline cartilage is rich in collagen type II and maintains nutrition through physiologic loading and unloading. 10 Once joint congruency is disturbed, however, successive joint degeneration results in osteoarthritis (OA) as adult cartilage has limited regenerative potential. 2 To prevent further degeneration and to improve clinical symptoms, numerous surgical treatment options for cartilage repair have been proposed. 11 Cell-based treatments such as autologous chondrocyte transplantation (ACI)12-15 and matrix-induced autologous chondrocyte implantation (MACI)16,17 require a 2-step procedure to initially harvest chondrocytes for incubation in the laboratory followed by implantation in a second surgery. Conversely, available 1-step procedures include osteochondral allograft implantation (OCA), which is limited in its availability of suitable allografts,18-20 bone marrow stimulation (microfracturing) for smaller OLTs,21-23 and autologous matrix-induced chondrogenesis (AMIC). 24 The AMIC technique comprises bone marrow stimulation of the subchondral bone with subsequent augmentation of a collagen type I/III bilayer membrane to contain the subchondral bleeding and provide a matrix for repair tissue maturation. Numerous studies reported good clinical improvement with high return to sport in the treatment of symptomatic OLTs,25-30 superior to simple bone marrow stimulation. 24
Postoperative magnetic resonance imaging (MRI) is the most important noninvasive method for evaluation of surgical procedures for cartilage repair.10,31-33 In 2004, Marlovits et al. 31 introduced the MOCART score (Magnetic Resonance Observation of Cartilage Repair Tissue) based on a standard knee MRI protocol including intermediate-weighted sequences for cartilage evaluation. Originally, the score was designed for the evaluation of cartilage repair tissue after microfracturing, ACI, or MACI in the knee. The score considers 9 radiologic variables (degree of repair and defect filling, integration to border tissue, surface of repair tissue, structure of repair tissue, subchondral lamina, subchondral bone, adhesions, synovitis) and its use has found broad implementation for AMIC assessment in OLT.25,28,29,34,35
While postoperative MRI assessment provides valuable information regarding structural integrity of the joint, the role of the MOCART score as a follow-up tool in cartilage repair of the ankle remains controversial.28,36,37,40 Recently, Schreiner et al. presented an updated version of the original MOCART score, the MOCART 2.0 score, 33 to mirror recent progressions in surgical and imaging techniques. The updated version overworked the variables, degree of defect filling from 50% into 25% increments, the integration to neighboring native cartilage, surface repair assessment in reference to repair length in place of depth, subchondral bone was changed to subchondral changes, which now includes signal intensity for edema in the bone marrow, cysts, or osteonecrosis. Meanwhile, the variables subchondral lamina, adhesions, and synovitis were excluded from the score with its points being reallocated to the new variable bony defect or bony overgrowth. While the purpose of this update was to enhance validity of postoperative MR imaging in cartilage repair, its clinical relevance is still to be determined.
Thus, the goal of the current study was to determine the role of MR imaging, namely, MOCART 1 and 2.0 scores, in the assessment of postoperative outcome after AMIC for the treatment of OLTs at a follow-up between 2 and 8 years. It was hypothesized that preoperative patient factors or OLT morphology are associated with postoperative MOCART scores, yet postoperative clinical outcome is not.
Materials and Method
Our ethics review board approved this study before initiation. Data are regularly collected and stored for patients undergoing elective surgery at our institution. This database was used to identify patients who underwent isolated cartilage repair with AMIC for focal osteochondral defects in the talus between October 2009 and August 2015. The surgical treatment with AMIC was indicated for symptomatic focal full-thickness chondral and OLTs. AMIC was contraindicated in patients with inflammatory arthritis and/or advanced osteoarthritis.
Patients were included in this study if they underwent isolated AMIC to the talus with postoperative MR imaging and had clinical outcome available, namely, the American Orthopaedic Foot and Ankle Society (AOFAS; 0-100 points) score 38 for ankle function and the Tegner score 39 (0-10 points) for sports activity. Exclusion criteria comprised any prior ankle joint surgery or concomitant ankle procedure, and if patients did not have MRI acquisition performed at the same date as clinical scores were obtained.
Clinical notes and operative reports were reviewed to determine patient’s age at the time of surgery, sex, body mass index (BMI), smoking status, duration of symptoms, concomitant bone grafting, defect size, and location.
Surgical Technique
All patients’ OLT were accessed via an open approach through a medial (n = 32) or lateral (n = 3) malleolar osteotomy.25,28,30
Undermined cartilage and flaps were sharply excised using a scalpel or curettage and cystic or necrotic bone lesions were debrided until vital bone tissue was visible. Next, the bone surface was microfractured by drilling with a K-wire (Ø 1.2 mm, DePuy Synthes, Oberdorf, Switzerland). When the extent of bone defect was cavernous or impressed the congruency, the semi-cylindrical joint surface of the talus was reconstructed with cancellous bone from the osteotomized tibia. Subsequently, the bilayer type I/III collagen matrix (Chondro-Gide, Geistlich Pharma AG, Wolhusen, Switzerland) was cut to fit the defect and placed on the lesion with the smooth side facing up. After checking if the lesion is filled up sufficiently, surgical fibrin glue was utilized to secure the membrane to the adjacent cartilage (Tissucol Duo S, Baxter International Inc., Deerfield, IL). Last, the joint was brought through full range of motion to ensure proper graft fixation, followed by open reduction and internal fixation of the malleolar osteotomy with two 2.5-mm titanium screws (medial) or compression plating (lateral) and standard wound closure.
MRI Assessment
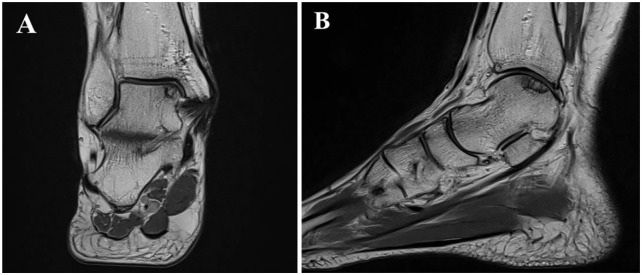
Postoperative MRIs were performed on a 1.5 T MAGNETOM Avanto Fit system (Siemens Healthcare, Erlangen, Germany) with a dedicated 8-channel foot and ankle coil using a musculoskeletal protocol incorporating coronal, sagittal and axial turbo spin-echo (TSE) intermediate-weighted sequences with and without fat saturation, using the Dixon technique, and a 3-mm slice thickness ( Fig. 1 ).
Figure 1.
Postoperative intermediate-weighted coronal (A) and sagittal (B) MRI depicting an osteochondral lesion on the medial talar dome accessed via a medial malleolar osteotomy.
For the present imaging study, the previously published and validated comprehensive MOCART 31 and MOCART 2.0 33 were utilized ranging from 0 to 100 points. Based on the original MOCART 31 scoring system comprising 9 radiologic variables, renewals of the MOCART 2.0 score 33 were the following: the degree of repair and defect filling is hypertrophic when exceeding 150% of the defect depth and incomplete filling is now assessed in 25% increments. The integration only accounts ingrowth into the neighboring native cartilage. Instead of regarding the depth of repair, the grading of surface irregularities is now referenced to the repaired length. The signal intensity division was retitled to normal signal, minor abnormal, and severely abnormal signal alterations. The subchondral bone score was revised to subchondral changes and added osteonecrosis as monitor. Last, the variables subchondral lamina, adhesions, and synovitis were removed and replaced by bony defect or bony overgrowth, reflecting a more detailed assessment of the bony lesion compared to the original score, which just rated the subchondral lamina as intact or not.
All MRIs were evaluated by a fellowship-trained musculoskeletal radiologist.
Statistical Analysis
Descriptive statistics were used to determine patient and lesion characteristics. All data were assessed for normality utilizing the Shapiro-Wilk test. Accordingly, continuous variables were analyzed with the independent t test. Point biserial and Pearson correlation, chi-square, and Fisher’s exact test were used to assess the relationship of MOCART subscales with patient’s age, sex, smoking status, BMI, duration of symptoms, bone grafting, lesion size and location, follow-up, and postoperative AOFAS and Tegner scores. Lesion size was calculated utilizing the ellipse formula for OLTs (sagittal length × coronal length × 0.79). 41 All statistical analyses were performed in SPSS for Mac (Version 23.0, SPSS Inc., Chicago, IL). Significance was set at P < 0.05.
Results
Thirty-five patients that underwent isolated AMIC for OLT at our institution with postoperative MRI and patient-reported outcomes were identified and included in this study. Mean postoperative MRI and clinical follow-up was obtained at 4.5 ± 1.8 years. Patient and lesion characteristics are presented in Table 1 .
Table 1.
Patient and Lesion Demographics.
| Patient and Lesion Characteristics | Patients (n = 35) |
|---|---|
| Age, years, mean ± SD | 34.4 ± 10.7 |
| Female sex, n (%) | 14 (40) |
| BMI, kg/m2, mean ± SD | 28.7 ± 5.4 |
| Smoker, n (%) | 17 (48.6) |
| DOS >12 months, n (%) | 27 (77.1) |
| Defect size, cm2, mean ± SD | 0.9 ± 0.6 |
| Defect location, medial/lateral | 32/3 |
| Bone grafting, yes/no | 27/8 |
SD = standard deviation; BMI = body mass index; DOS = duration of symptoms.
At final follow-up, mean AOFAS score was 92.63 ± 8.3 and patients reported a mean Tegner score of 5.1 ± 1.8, which significantly increased from 3.7 ± 2.0 (P = 0.002) preoperatively. Both MRI assessments were significantly associated with each other with a mean MOCART 1 score of 59.0 ± 14.9 compared to a mean MOCART 2.0 score of 65.1 ± 13.9 (r = 0.885; P < 0.001). In fact, subscales of both MOCART scores were strongly correlated with each other, except “signal intensity” (P < 0.001 and P = 0.096, respectively; Table 2 ).
Table 2.
Bivariate Correlations of Postoperative MOCART 1 and MOCART 2.0 Subscales and Total Score.
| MOCART 1 | Mean ± SD | MOCART 2.0 | Mean ± SD | r | P Value |
|---|---|---|---|---|---|
| Filling of defect | 16.9 ± 3.4 | Volume fill of cartilage defect | 19.1 ± 2.8 | 0.693 | <0.001 |
| Integration to border zone | 13.0 ± 4.1 | Integration to border zone | 13.9 ± 2.5 | 0.946 | <0.001 |
| Surface of repair tissue | 6.4 ± 3.1 | Surface of repair tissue | 6.9 ± 3.0 | 0.813 | <0.001 |
| Structure of repair tissue | 1.4 ± 2.3 | Structure of repair tissue | 2.9 ± 4.5 | 1 | <0.001 |
| Signal intensity of repair tissue | 14.1 ± 7.2 | Signal intensity of repair tissue | 10.0 ± 3.2 | 0.286 | 0.096 |
| Subchondral lamina | 0.9 ± 1.9 | Bony defect/bony overgrowth | 3.7 ± 3.7 | 0.575 | <0.001 |
| Subchondral bone | 0.4 ± 1.4 | Subchondral changes | 8.7 ± 5.9 | 0.510 | 0.002 |
| Adhesion | 3.1 ± 2.5 | — | — | — | — |
| Effusion | 2.7 ± 2.5 | — | — | — | — |
| Total | 59.0 ± 14.9 | Total | 65.1 ± 13.9 | 0.885 | <0.001 |
Analyzing the influence of preoperative factors on postoperative MRI assessment, osteochondral lesion size was the only preoperative factor that showed significant correlation with postoperative MOCART 1 and 2.0 total scores (P = 0.006 and P = 0.004, respectively). Bone grafting was the only preoperative factor significantly associated with a MOCART 1 subscale (“signal intensity”). Both age and defect size showed significant inverse correlations with MOCART 2.0 subscales (P < 0.05; Table 3 ).
Table 3.
Significant Bivariate Correlations of Preoperative Patient and Lesion Parameters With Postoperative MOCART 1 and 2.0 Total Scores and Subscales.
| Correlation Coefficient | P Value | |
|---|---|---|
| Total MOCART 1 | ||
| Defect size | −0.453 | 0.006 |
| Signal intensity of repair tissue | ||
| Bone grafting | 0.530 | 0.038 |
| Total MOCART 2.0 | ||
| Defect size | −0.477 | 0.004 |
| Volume fill of cartilage defect | ||
| Age | −0.401 | 0.017 |
| Surface of repair tissue | ||
| Defect size | −0.421 | 0.012 |
| Signal intensity of repair tissue | ||
| Defect size | −0.354 | 0.037 |
Stratifying patients based on their follow-up, patients with a shorter follow-up (less than 4.5 years) showed a significantly higher MOCART 1 score than patients with a follow-up longer than 4.5 years (64.7 ± 10.8 vs. 52.9 ± 16.6, P = 0.02). Patients with a shorter follow-up also exhibited a trend toward better MOCART 2.0 scores than patients with longer follow up (69.4 ± 12.4 vs. 60.6 ± 14.3, P = 0.058). Specifically, MOCART 1 and 2.0 “signal intensity” subscale was significantly better in patients with shorter follow-up (P = 0.016 and P = 0.031, respectively). Furthermore, MOCART 1 and 2.0 “structure of repair tissue” subscale showed a trend toward better results in patients with shorter follow-up (both, P = 0.06).
When correlating postoperative MRI scores with clinical outcome, neither MOCART 1 nor 2.0 total or subscale scores were related to AOFAS or Tegner scores (n.s.).
Discussion
The key finding of the study presented herein is that MOCART and MOCART 2.0 scores strongly correlate with each other but do not show a statistically significant association with clinical outcome after isolated AMIC for the treatment of symptomatic OLTs. Interestingly, defect size was the only preoperative factor that was correlated with both postoperative total MOCART scores, which both seem to decline with prolonged follow-up.
While no direct comparison between the MOCART scores or an evaluation of the relationship between MOCART 2.0 and postoperative clinical outcome after cartilage repair in the ankle has been established yet, some studies have assessed postoperative MR imaging after AMIC and have investigated its association with patient-reported clinical outcome. Kubosch et al. 28 presented a case series of 17 patients treated with the open AMIC procedure for medial OLTs. At a mean follow-up of 39.5 ± 18.4 months, postoperative MOCART scores averaged 52.7 ± 15.9 with AOFAS scores averaging 82.6 ± 13.4. Conversely to the presented results, the authors observed a statistically significant correlation between postoperative MOCART and AOFAS score (rho = 0.574, P = 0.04). Moreover, osteochondral lesion size ≥3 cm3 was significantly associated with inferior AOFAS scores in the studied cohort, a factor that was inversely correlated with MOCART and MOCART 2.0 scores in the current study.
In a recently published study by Weigelt et al., 25 the authors reported good to excellent clinical outcome with high return to sport after AMIC for OLTs in 33 patients with a mean MOCART score of 60.6 ± 21.2. Correlating solely total MOCART scores, and not its subscales, to postoperative clinical outcome, no significant association was found. A similar finding was reported by Albano et al., 34 who evaluated 16 patients at a mean follow-up of 30 ± 16.9 months after AMIC for OLT. MRI acquisition was obtained at 12 and 24 months postoperatively and correlated with clinical outcome, assessed by VAS and AOFAS. Though MOCART scores increased significantly from 41.9 ± 14.6 at 12 months to 51.9 ± 11.6 at 24 months, their change did not show any significant association with the clinical improvement measured by the AOFAS.
While none of these studies investigated the relationship between MOCART subscales and clinical outcome, they do partially reflect the findings of the current study that neither MOCART total nor subscale scores are related with clinical outcome after AMIC for OLT. In a report published by de Windt et al., the authors systematically reviewed the evidence whether morphological MRI is reliable in predicting clinical outcome after cartilage repair in the knee. Assessing a total of 32 studies, they concluded that a majority of MRI parameters including the MOCART score did not show a significant correlation with clinical outcome, yet highlighting the fact that developing more advanced imaging techniques are warranted in the field of cartilage repair. 42
On the other hand, the absence of correlation between MOCART and clinical outcome might be rooted in the particular postoperative time period MRI examinations were obtained. Ackermann et al. 43 presented a cohort study of 67 patients treated with ACI for cartilage defects in the knee. Analyzing the effect of chondrocytic gene expression on postoperative MR imaging and clinical outcome, the authors did not see any effect of increased chondrocytic gene expression on MOCART scores at final follow-up. When evaluating postoperative MRIs that were obtained at a follow-up between 12 and 30 months, however, increased gene expression was significantly associated with greater degree of defect filling, better integration at the border zone, and decreased effusion. It was theorized that 12 to 30 months might be the ideal time period for MRI evaluation of the repair tissue after ACI to the knee. They reasoned that ACI graft maturation requires 12 to 24 months to complete, 44 but graft aging with decreasing MOCART scores and general deterioration of the joint starts approximately after 30 months of follow-up. 45 Hence, 12 to 30 months represents a time period were a reliable evaluation of the repair tissue could be determined. Projecting this finding to the AMIC procedure, Gobbi et al., 46 in a histological study, investigated the repair tissue after AMIC for OLTs. The study found that AMIC produced hyaline-like repair tissue in patients at 13.5 months postoperatively, suggesting complete maturation of the repair tissue. In the study presented herein, MRIs were obtained at a mean follow-up of 4.5 years with decreasing MOCART scores over time. This follow-up might explain the generally rather low overall MOCART scores as well as the absent association between MR findings and clinical outcome. Yet, follow-up did not affect the association between MOCART and clinical outcome in this study. However, future longitudinal studies are warranted to investigate if the clinical effect of early low MOCART scores might just occur at later time points postoperatively. Until then, it remains questionable if timing plays a role in evaluating repair tissue of OLT treated with AMIC as the minimum follow-up was 2 years and graft deterioration might have already begun. Consequently, postoperative MR assessment with MOCART seems to not provide any further valuable information regarding clinical outcome after 2 years in patients that underwent isolated AMIC for OLTs who do not experience any symptoms that require further MR imaging evaluation to assess joint integrity.
However, the more detailed increments of the MOCART 2.0 might have slightly increased some subscale sensitivity. In fact, volume fill of cartilage defect inversely correlated with patient’s age, meaning that younger patients presented with a more sufficient filling of the defect than patients of older age. This finding matches the report of Pestka et al., 47 who analyzed cell quality in an in vitro examination of ACI cell cultivations. A total of 252 ACI patient samples were assessed for the cartilage relevant surface marker CD44 and the cartilage-specific differentiation markers aggrecan and collagen type II. The study revealed that patients younger than 20 years showed significantly higher expression rates of all 3 markers compared to older patients suggesting better cell quality. This might have contributed to the result seen in the current study; however, it will be of interest to see if similar observations can be made in cell-based therapies such as ACI and MACI utilizing the MOCART 2.0.
Last, defect size was the only preoperative factor that correlated with postoperative MOCART and MOCART 2.0 scores. While microfracturing is still referred to as the gold standard for lesions sizes up to 1.5 cm2, 48 AMIC has shown to result in favorable outcome in smaller as well as larger lesions without defect size being correlated with the postoperative clinical outcome. 25 Becher et al. treated 15 patients with mircrofracturing for OLTs and, similar to the results seen in the current study, found defect size to be a factor significantly associated with postoperative MOCART scores with higher scores found in patients with smaller lesions, yet this did not translate into improved clinical outcomes. 49
The authors acknowledge the limitations of this study. The study was retrospective in its design with unavailable preoperative clinical scores. Thus, clinical improvement can only be assumed with a postoperative AOFAS score averaging over 92 points and postoperative Tegner scores showing a significant improvement compared to the retrospectively collected preoperative Tegner scores. Further-more, both MOCART scores were originally designed for evaluating cartilage repair in the knee, yet its application in the assessment of cartilage repair in the ankle is widely accepted.25,28,29,34,35,37,50
Conclusion
In summary, osteochondral lesion size is associated with postoperative MOCART scores in patients treated with AMIC for OLTs, with decreasing MOCART scores over time. Yet, clinical outcome does not correlate with any MRI parameter assessed by MOCART or MOCART 2.0. Thus, assessment of either MOCART score seems to have no significant role in the postoperative treatment of asymptomatic patients that underwent AMIC for OLTs.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval for this study was obtained from the cantonal ethics committee of Zurich (BASEC-Nr. 2019-02025).
Informed Consent: Written informed consent was obtained from all subjects before the study.
ORCID iDs: Fabio A. Casari  https://orcid.org/0000-0002-9530-4861
https://orcid.org/0000-0002-9530-4861
Christoph Germann  https://orcid.org/0000-0001-9273-0032
https://orcid.org/0000-0001-9273-0032
References
- 1. Hintermann B, Regazzoni P, Lampert C, Stutz G, Gächter A. Arthroscopic findings in acute fractures of the ankle. J Bone Joint Surg Br. 2000;82(3):345-51. [DOI] [PubMed] [Google Scholar]
- 2. Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64(3):460-6. [PubMed] [Google Scholar]
- 3. Ackermann J, Fraser EJ, Murawski CD, Desai P, Vig K, Kennedy JG. Trends of concurrent ankle arthroscopy at the time of operative treatment of ankle fracture: a national database review. Foot Ankle Spec. 2015;9(2):107-12. [DOI] [PubMed] [Google Scholar]
- 4. Flick AB, Gould N. Osteochondritis dissecans of the talus (transchondral fractures of the talus): review of the literature and new surgical approach for medial dome lesions. Foot Ankle. 1985;5(4):165-85. [DOI] [PubMed] [Google Scholar]
- 5. O’Loughlin PF, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med. 2010;38(2):392-404. [DOI] [PubMed] [Google Scholar]
- 6. Holmer P, Søndergaard L, Konradsen L, Nielsen PT, Jorgensen LN. Epidemiology of sprains in the lateral ankle and foot. Foot Ankle Int. 1994;15(2):72-4. [DOI] [PubMed] [Google Scholar]
- 7. DiGiovanni BF, Fraga CJ, Cohen BE, Shereff MJ. Associated injuries found in chronic lateral ankle instability. Foot Ankle Int. 2000;21(10):809-15. [DOI] [PubMed] [Google Scholar]
- 8. Ferkel RD, Chams RN. Chronic lateral instability: arthroscopic findings and long-term results. Foot Ankle Int. 2007;28(1_suppl):24-31. [DOI] [PubMed] [Google Scholar]
- 9. Bonnel F, Toullec E, Mabit C, Tourné Y, Sofcot. Chronic ankle instability: biomechanics and pathomechanics of ligaments injury and associated lesions. Orthop Traumatol Surg Res. 2010;96(4):424-32. [DOI] [PubMed] [Google Scholar]
- 10. Imhof H, Nöbauer-Huhmann IM, Krestan C, Gahleitner A, Sulzbacher I, Marlovits S, et al. MRI of the cartilage. Eur Radiol. 2002;12(11):2781-93. [DOI] [PubMed] [Google Scholar]
- 11. Ambra LF, de Girolamo L, Mosier B, Gomoll AH. Review: interventions for cartilage disease: current state-of-the-art and emerging technologies. Arthritis Rheumatol. 2017;69(7):1363-73. [DOI] [PubMed] [Google Scholar]
- 12. Imhoff AB, Paul J, Ottinger B, Wortler K, Lammle L, Spang J, et al. Osteochondral transplantation of the talus: long-term clinical and magnetic resonance imaging evaluation. Am J Sports Med. 2011;39(7):1487-93. [DOI] [PubMed] [Google Scholar]
- 13. Giannini S, Vannini F, Buda R. Osteoarticular grafts in the treatment of OCD of the talus: mosaicplasty versus autologous chondrocyte transplantation. Foot Ankle Clin. 2002;7(3):621-33. [DOI] [PubMed] [Google Scholar]
- 14. Flynn S, Ross KA, Hannon CP, Yasui Y, Newman H, Murawski CD, et al. Autologous osteochondral transplantation for osteochondral lesions of the talus. Foot Ankle Int. 2016;37(4):363-72. [DOI] [PubMed] [Google Scholar]
- 15. Kim YS, Park EH, Kim YC, Koh YG, Lee JW. Factors associated with the clinical outcomes of the osteochondral autograft transfer system in osteochondral lesions of the talus: second-look arthroscopic evaluation. Am J Sports Med. 2012;40(12):2709-19. [DOI] [PubMed] [Google Scholar]
- 16. Bellido PC, Wadhwani J, Monzo EG. Matrix-induced autologous chondrocyte implantation grafting in osteochondral lesions of the talus: evaluation of cartilage repair using T2 mapping. J Orthop. 2019;16(6):500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aurich M, Bedi HS, Smith PJ, Rolauffs B, Muckley T, Clayton J, et al. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation: early clinical and magnetic resonance imaging results. Am J Sports Med. 2011;39(2):311-9. [DOI] [PubMed] [Google Scholar]
- 18. Haene R, Qamirani E, Story RA, Pinsker E, Daniels TR. Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg Am. 2012;94(12):1105-10. [DOI] [PubMed] [Google Scholar]
- 19. Raikin SM. Fresh osteochondral allografts for large-volume cystic osteochondral defects of the talus. J Bone Joint Surg Am. 2009;91(12):2818-26. [DOI] [PubMed] [Google Scholar]
- 20. Heida KA, Jr, Tihista MC, Kusnezov NA, Dunn JC, Orr JD. Outcomes and predictors of postoperative pain improvement following particulated juvenile cartilage allograft transplant for osteochondral lesions of the talus. Foot Ankle Int. 2020;41(5):572-81. [DOI] [PubMed] [Google Scholar]
- 21. Ferkel RD, Zanotti RM, Komenda GA, Sgaglione NA, Cheng MS, Applegate GR, et al. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36(9):1750-62. [DOI] [PubMed] [Google Scholar]
- 22. Schuman L, Struijs PA, van Dijk CN. Arthroscopic treatment for osteochondral defects of the talus. Results at follow-up at 2 to 11 years. J Bone Joint Surg Br. 2002;84(3):364-8. [DOI] [PubMed] [Google Scholar]
- 23. van Bergen CJ, Kox LS, Maas M, Sierevelt IN, Kerkhoffs GM, van Dijk CN. Arthroscopic treatment of osteochondral defects of the talus: outcomes at eight to twenty years of follow-up. J Bone Joint Surg Am. 2013;95(6):519-25. [DOI] [PubMed] [Google Scholar]
- 24. Volz M, Schaumburger J, Frick H, Grifka J, Anders S. A randomized controlled trial demonstrating sustained benefit of autologous matrix-induced chondrogenesis over microfracture at five years. Int Orthop. 2017;41(4):797-804. [DOI] [PubMed] [Google Scholar]
- 25. Weigelt L, Hartmann R, Pfirrmann C, Espinosa N, Wirth SH. Autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: a clinical and radiological 2- to 8-year follow-up study. Am J Sports Med. 2019;47(7):1679-86. [DOI] [PubMed] [Google Scholar]
- 26. Wiewiorski M, Leumann A, Buettner O, Pagenstert G, Horisberger M, Valderrabano V. Autologous matrix-induced chondrogenesis aided reconstruction of a large focal osteochondral lesion of the talus. Arch Orthop Trauma Surg. 2011;131(3):293-6. [DOI] [PubMed] [Google Scholar]
- 27. Wiewiorski M, Werner L, Paul J, Anderson AE, Barg A, Valderrabano V. Sports activity after reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med. 2016;44(10):2651-8. [DOI] [PubMed] [Google Scholar]
- 28. Kubosch EJ, Erdle B, Izadpanah K, Kubosch D, Uhl M, Sudkamp NP, et al. Clinical outcome and T2 assessment following autologous matrix-induced chondrogenesis in osteochondral lesions of the talus. Int Orthop. 2016;40(1_suppl):65-71. [DOI] [PubMed] [Google Scholar]
- 29. Valderrabano V, Miska M, Leumann A, Wiewiorski M. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med. 2013;41(3):519-27. [DOI] [PubMed] [Google Scholar]
- 30. Behrens P. Matrixgekoppelte Mikrofrakturierung. Arthros-kopie. 2005;18(3):193-7. [Google Scholar]
- 31. Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52(3):310-9. [DOI] [PubMed] [Google Scholar]
- 32. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1_suppl):16-23. [DOI] [PubMed] [Google Scholar]
- 33. Schreiner MM, Raudner M, Marlovits S, Bohndorf K, Weber M, Zalaudek M, et al. The MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) 2.0 Knee Score and Atlas. Cartilage. Epub 2019 August 17. doi: 10.1177/1947603519865308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Albano D, Martinelli N, Bianchi A, Messina C, Malerba F, Sconfienza LM. Clinical and imaging outcome of osteochondral lesions of the talus treated using autologous matrix-induced chondrogenesis technique with a biomimetic scaffold. BMC Musculoskelet Disord. 2017;18(1_suppl):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Galla M, Duensing I, Kahn TL, Barg A. Open reconstruction with autologous spongiosa grafts and matrix-induced chondrogenesis for osteochondral lesions of the talus can be performed without medial malleolar osteotomy. Knee Surg Sports Traumatol Arthrosc. 2019;27(9):2789-95. [DOI] [PubMed] [Google Scholar]
- 36. Albano D, Martinelli N, Bianchi A, Giacalone A, Sconfienza LM. Evaluation of reproducibility of the MOCART score in patients with osteochondral lesions of the talus repaired using the autologous matrix-induced chondrogenesis technique. Radiol Med. 2017;122(12):909-17. [DOI] [PubMed] [Google Scholar]
- 37. Apprich S, Trattnig S, Welsch GH, Noebauer-Huhmann IM, Sokolowski M, Hirschfeld C, et al. Assessment of articular cartilage repair tissue after matrix-associated autologous chondrocyte transplantation or the microfracture technique in the ankle joint using diffusion-weighted imaging at 3 Tesla. Osteoarthritis Cartilage. 2012;20(7):703-11. [DOI] [PubMed] [Google Scholar]
- 38. Van Lieshout EMM, De Boer AS, Meuffels DE, Hoed PTD, Van der Vlies CH, Tuinebreijer WE, et al. American Orthopaedic Foot and Ankle Society (AOFAS) Ankle-Hindfoot Score: a study protocol for the translation and validation of the Dutch language version. BMJ Open. 2017;7:e012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43-9. [PubMed] [Google Scholar]
- 40. Martinelli N, Albano D, Prati ABC, Antonino G, Malerba F, Sconfienza L. MOCART score is not a feasible tool for the assessment of osteochondral lesions of the talus. Foot Ankle Orthop. 2017;2(3):2473011417S2473000285. [Google Scholar]
- 41. Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37(10):1974-80. [DOI] [PubMed] [Google Scholar]
- 42. de Windt TS, Welsch GH, Brittberg M, Vonk LA, Marlovits S, Trattnig S, et al. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am J Sports Med. 2013;41(7):1695-702. [DOI] [PubMed] [Google Scholar]
- 43. Ackermann J, Merkely G, Mestriner AB, Shah N, Gomoll AH. Increased chondrocytic gene expression is associated with improved repair tissue quality and graft survival in patients after autologous chondrocyte implantation. Am J Sports Med. 2019;47(12):2919-26. [DOI] [PubMed] [Google Scholar]
- 44. Niethammer TR, Safi E, Ficklscherer A, Horng A, Feist M, Feist-Pagenstert I, et al. Graft maturation of autologous chondrocyte implantation: magnetic resonance investigation with T2 mapping. Am J Sports Med. 2014;42(9):2199-204. [DOI] [PubMed] [Google Scholar]
- 45. Rosa D, Balato G, Ciaramella G, Soscia E, Improta G, Triassi M. Long-term clinical results and MRI changes after autologous chondrocyte implantation in the knee of young and active middle aged patients. J Orthop Traumatol. 2016;17(1_suppl):55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. Cartilage. 2011;2(3):286-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pestka JM, Schmal H, Salzmann G, Hecky J, Südkamp NP, Niemeyer P. In vitro cell quality of articular chondrocytes assigned for autologous implantation in dependence of specific patient characteristics. Arch Orthop Trauma Surg. 2011;131(6):779-89. [DOI] [PubMed] [Google Scholar]
- 48. Dahmen J, Lambers KTA, Reilingh ML, van Bergen CJA, Stufkens SAS, Kerkhoffs G. No superior treatment for primary osteochondral defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):2142-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Becher C, Zuhlke D, Plaas C, Ewig M, Calliess T, Stukenborg-Colsman C, et al. T2-mapping at 3 T after microfracture in the treatment of osteochondral defects of the talus at an average follow-up of 8 years. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2406-12. [DOI] [PubMed] [Google Scholar]
- 50. Toale J, Shimozono Y, Mulvin C, Dahmen J, Kerkhoffs G, Kennedy JG. Midterm outcomes of bone marrow stimulation for primary osteochondral lesions of the talus: a systematic review. Orthop J Sports Med. 2019;7(10):2325967119879127. [DOI] [PMC free article] [PubMed] [Google Scholar]