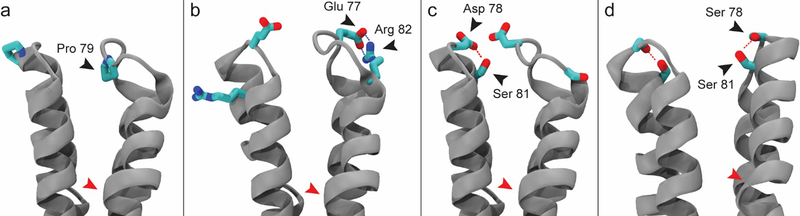
Figure 6.
Molecular dynamics simulations reveal variations in secondary structure and stabilizing interactions in dimer spike tips. (a) The left half-dimer exhibits the fully helical spike tip conformation. The right half-dimer exhibits the partially helical state, where Pro79 to Arg82 of helix 4a are unfolded. (b) A salt bridge between Glu77 and Asp82 can stabilize the partial helical state and impair the ability of helix 4a to recover. (c) Asp78 stabilizes helix 4a by capping its N-terminus and by forming a strong hydrogen bond with Ser81. (d) Ser78 can fulfill the role of N-cap but does not consistently hydrogen bond with Ser81. Hydrogen atoms omitted for clarity. Location of the spike hinge indicated by red arrow.