Figure 4. Coculture of peripheral blood MNCs and MCs activates both cell types.
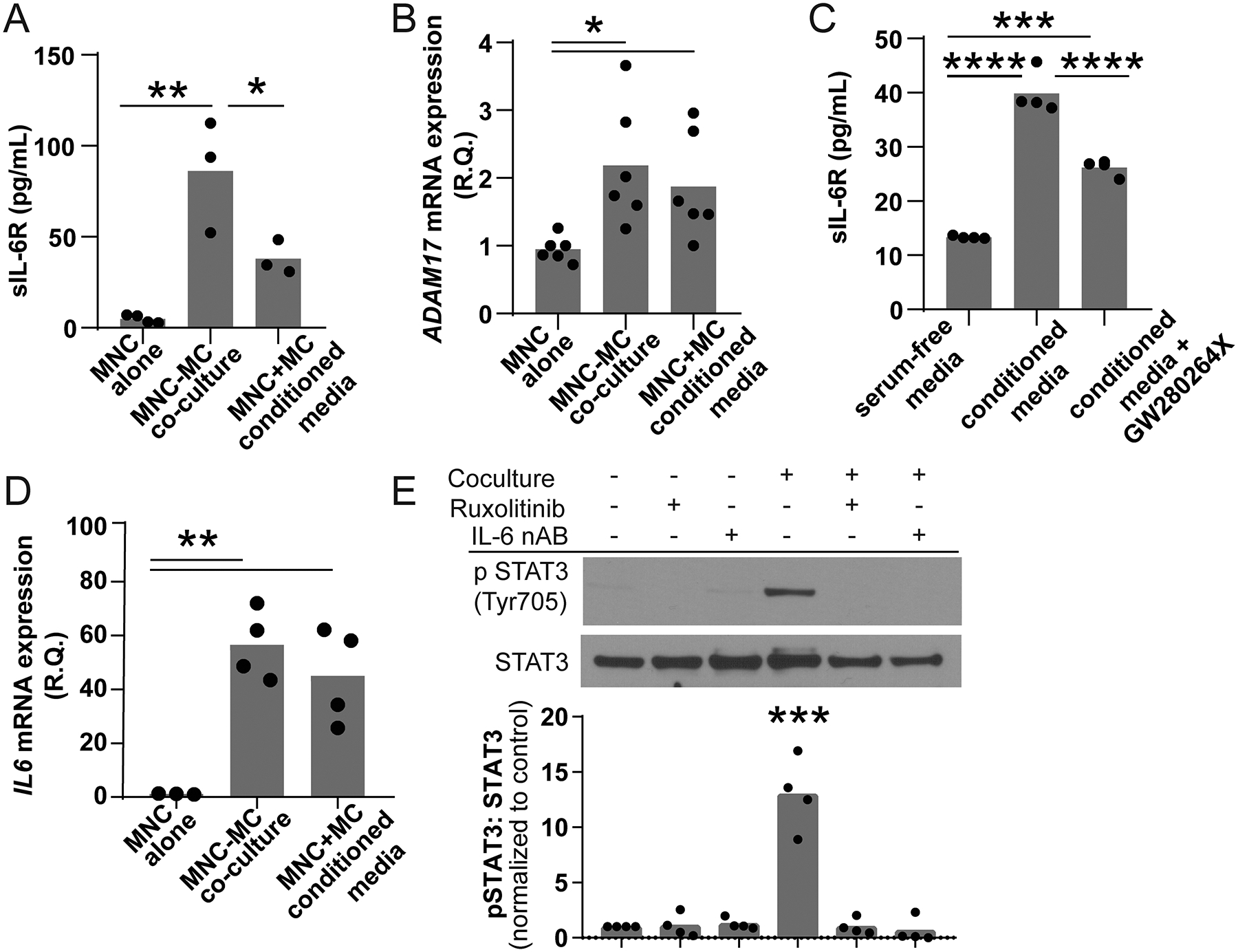
Peripheral blood MNCs (top chamber) were grown in the presence or absence of MCs (bottom chamber) in a transwell containing serum free media for 24 hours. (A) MNCs cocultured with MC produced significantly higher levels of sIL-6R (86.20±30.82, n=3) compared to PBMCs grown alone (4.88±2.20, n=4, **p<0.01). MNCs treated with conditioned media also had an increase in sIL-6R generation that was lower in the coculture model (37.96±9.30, n=3, *p<0.05). (B) Both MNC cocultured with MCs and treated with MC-conditioned media had significant upregulation of ADAM17 mRNA (0.95±0.19 vs 1.875±0.77 vs 2.183±0.9, n=4, **p<0.001). (C) The addition of the ADAM17 inhibitor GW280264X reduced the amount of sIL-6R stimulated by MC-conditioned media by approximately 51% suggesting that membrane shedding was a significant source of media sIL-6R (13.32+0.25 vs 39.85+3.19 vs 26.22+1.47, n=4, ***p<0.001 ****p<0.0001). (D) The presence of MCs also induced a 50-fold increase in MNC IL-6 mRNA expression (1.158±0.14 versus 56.50±12.92, n=3–4, p=0.0008). (E) The lysates of MCs grown in the presence or absence of MNCs, 10 nM ruxolitinib and/or 0.1 μg/mL IL-6 neutralizing antibody were probed for phospho-STAT3 Tyr705. Coculture stimulated robust STAT3 phosphorylation consistent with IL-6 trans-signaling (*** p<0.001, n=4). This phosphorylation was blocked by inhibition of JAK and neutralization of IL-6.
