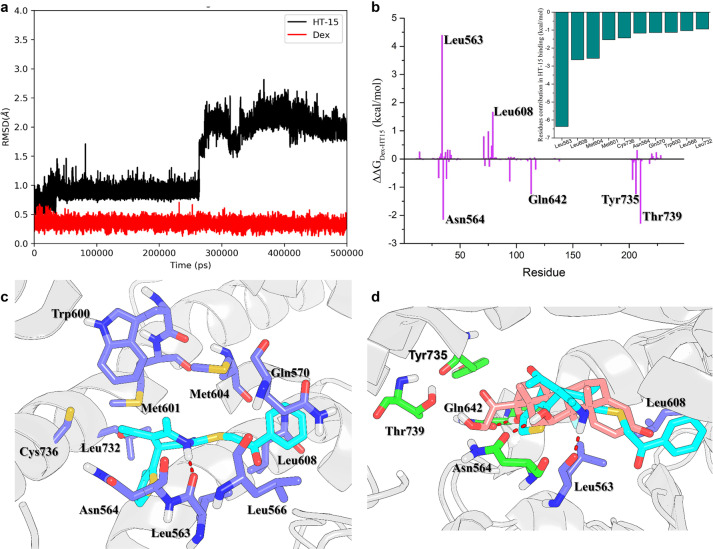
Fig. 4. The MD simulations and key residues in the GR LBD for the binding of HT-15.
a The time evolution of the RMSDs of the heavy atoms of HT-15 and Dex. b The energy differences between Dex and HT-15. The small figure is the 10 top-ranked residues in the GR LBD responsible for the binding of HT-15 predicted by MM/GBSA. c The structural analysis of the 10 top-ranked residues to the binding of HT-15. d Alignment of the representative structures of Dex (pink) and HT-15 (cyan) bound to the GR LBD. The residues that form stronger interaction with Dex than with HT-15 are highlighted in green. The residues that form stronger interaction with HT-15 than with Dex are highlighted in violet.