Table 1. Pharmacological properties of clinical PI3K inhibitors.
PI3K inhibitors that have reached late phases of clinical development and/or have been approved by the FDA for the treatment of cancer are listed.
| In vitro IC50 (nM) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PI3K inhibitor | Company | Structure | PI3Kα | PI3Kβ | PI3K | PI3Kγ | Clinical | Dose | t1/2 (h) | Registration trial | Ref |
| Alpelisib | Novartis |
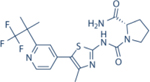
|
4.6 | 1156 | 290 | 250 | Approved for HR+/Her2- mBC in combination with Fulvestrant | Oral; 300 mg daily | 8 | NCT02437318 | 71–73 |
| Buparlisib | Novartis |
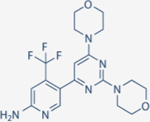
|
52 | 166 | 116 | 262 | Discontinued during Phase III | Oral; 100 mg daily | 40 | NCT01633060 | 58,121 |
| Copanlisib | Bayer |
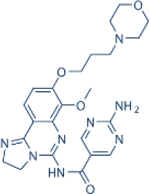
|
0.5 | 3.7 | 0.7 | 6.4 | Approved for relapsed follicular lymphoma | IV; 60 mg three times a month | 39.1 | NCT01660451 | 68,69 |
| Duvelisib | Verastem |
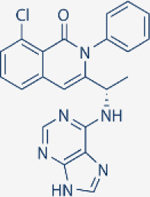
|
1602 | 85 | 2.5 | 27 | Approved for relapsed follicular lymphoma, Chronic lymphocytic leukemia; small lymphocytic leukemia | Oral; 25 mg twice daily | 4.7 | NCT02004522 | 81 |
| Idelalisib | Gilead |
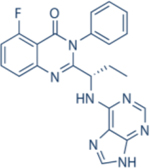
|
8600 | 4000 | 19 | 110 | Approved for relapsed follicular lymphoma, Chronic lymphocytic leukemia; small lymphocytic leukemia | Oral; 150 mg twice daily | 8.2 | NCT01282424 | 122,123 |
| Inavolisib | Genentech |
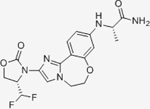
|
0.038 | N/A | N/A | N/A | Ongoing Phase III in combination with Fulvestrant and palbociclib for HR+/Her2- mBC | Oral; 9 mg daily | 18 | NCT04191499 | 124,125 |
| Taselisib | Genentech |
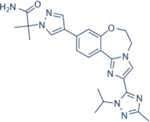
|
0.029 | 8 | 0.12 | 0.97 | Discontinued during Phase III | Oral; 4 mg daily | 39.3 | NCT02340221 | 76,77,94 |
| Umbrasilib | TG Therapeutics |
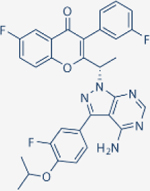
|
>10000 | >10000 | 6.2 | 1400 | Approved for relapsed follicular lymphoma and marginal zone lymphoma | Oral; 800 mg daily | 91 | NCT02793583 | 82 |
