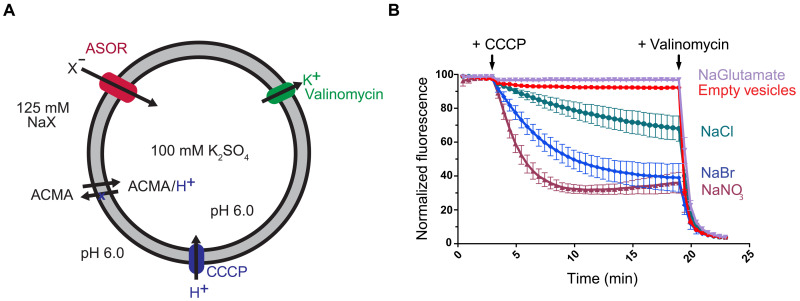
Fig. 1. Anion flux through purified ASOR.
(A) Schematic of the fluorescence-based flux assay. Purified ASOR (TMEM206) was reconstituted into liposomes. Liposomes, which were filled with potassium sulfate, were then diluted into various sodium salts (NaX) to establish ion gradients. Na+, K+, and sulfate are not readily permeable through the ASOR channel (5). Anion influx through the channel (into the liposome) produces a negative electric potential within the liposomes that drives the uptake of protons through an ionophore (CCCP) and quenches the fluorescence of a pH indicator (ACMA). (B) Flux measurements. Time-dependent decreases in fluorescence are indicative of anion flux. Glutamate was not detectably permeant. “Empty vesicles” indicate liposomes without protein. Arrows indicate additions of CCCP and the K+ ionophore valinomycin. Valinomycin, which causes efflux of K+ from the vesicles, was used to establish a fluorescence baseline and to confirm the integrity of liposomes. Fluorescence values were normalized by dividing by the value before CCCP addition and were within ±10% among the experiments. Because ACMA is sensitive to pH, we were not able to use this assay to study the pH-dependent activation of the reconstituted channel.