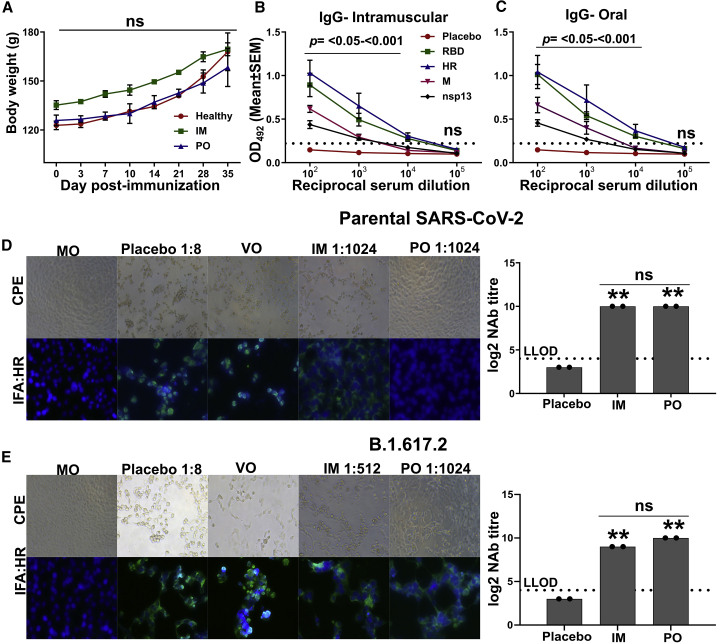
Figure 4.
Analysis of humoral and cross-protective neutralizing antibodies in hamster
Syrian hamsters were immunized via intramuscular and oral routes. The intramuscular injection consisted of a single dose of 2 × 107 CFU, whereas the oral route of administration consisted of two doses of 2 × 108 CFU at a 2-week interval. The IgG and NAb titers were evaluated 3 weeks after the final immunization. (A) Body weights of hamster post-immunization. Data denote two biologically independent hamsters per group. (B and C) IgG response in sera of hamsters immunized through the intramuscular and oral routes, respectively. Data were derived from six biologically independent hamsters per group. Dotted lines indicate the cutoff value determined by multiplying the mean OD492 values of healthy sera by 2.1. (D and E) Representative CPE and IFA images showing the viral replication by detecting the expression of HR protein of SARS-CoV-2. One of the wells of a serum dilution recorded as NAb titer is shown. NAb titer quantified by CPE and IFA analysis is presented in the right panel. (D) SARS-CoV-2 parental strain and (E) SARS-CoV-2 (B)1.617.2 variant. The dashed line in (D) and (E) represents the lower limit of detection (LLOD). MO, medium-only control; VO, virus-only control. Data in (A) were analyzed by two-way ANOVA using Bonferroni post test. Data in (B) and (C) were analyzed by unpaired Student's t test. Data in (D and E) were analyzed by one-way ANOVA using Bonferroni's multiple comparison test. The data points represent the individual value from each animal and error bars denote the SEM at 95% CI. ns, p > 0.05; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.