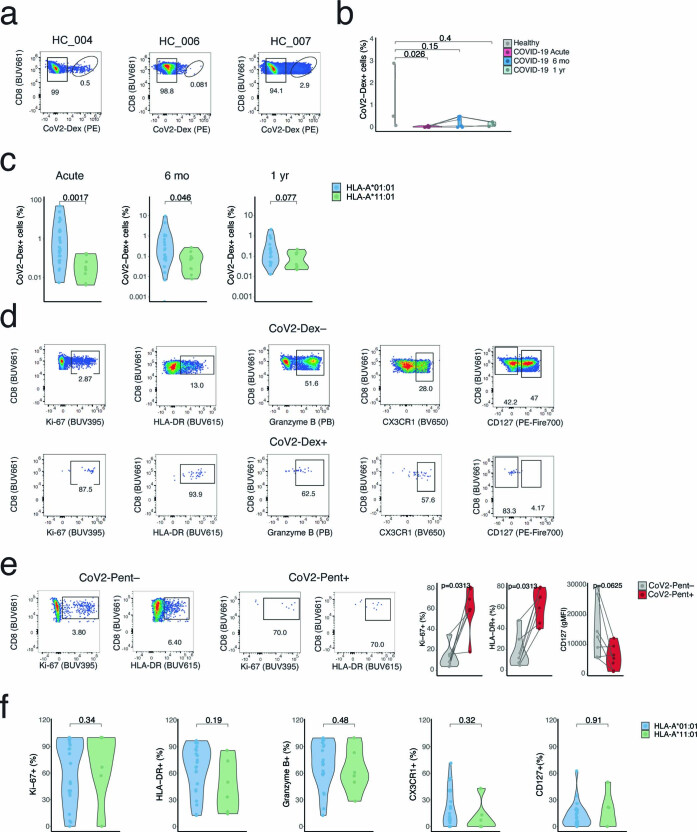
Extended Data Fig. 2. HLA-A*24:02 dextramer staining and CoV2-Dex+ cell frequency and phenotype based on HLA.
a, Representative plots of CoV2-Dex (HLA-A*24:02) staining in healthy donors. b, Percentage of CoV2-Dex+ cells in HLA-A*24:02 healthy donors and COVID-19 patients in acute infection and six months and one year after infection. Dots represent independent donors at each timepoint (n = 3 healthy, n = 6 acute, n = 5 six months, n = 3 one year). Gray lines connect individual donors sampled at different timepoints. c, Percentage of CoV2-Dex+ cells in HLA-A*01:01 and HLA-A*11:01 in acute infection and six months and one year after infection (HLA-A*01:01 n = 26 and HLA-A*11:01 n = 10 acute, HLA-A*01:01 n = 21 and HLA-A*11:01 n = 9 six months, HLA-A*01:01 n = 20 and HLA-A*11:01 n = 9 one year). d, Gating strategy for phenotypical analysis of CoV2-Dex+ compared to CoV2-Dex– cells. e, Gating strategy for phenotypical analysis of CoV2-Pent+ compared to CoV2-Pent– cells and frequency of Ki-67+, HLA-DR+ and CD127 levels in CoV2-Pent– (gray) and CoV2-Pent+ (red) cells in acute COVID-19 (n = 7). f, Frequency of Ki-67+, HLA-DR+, granzyme B+, CX3CR1+ and CD127+ cells in CoV2-Dex+ cells in patients with an HLA-A*01:01 versus HLA-A*11:01 allele (HLA-A*01:01 n = 22 and HLA-A*11:01 n = 6) in the acute phase. b, c, and f, P-values were calculated with a Mann-Whitney-Wilcoxon test. e, P-values were calculated with a Wilcoxon signed-rank test. All tests were performed two-sided.