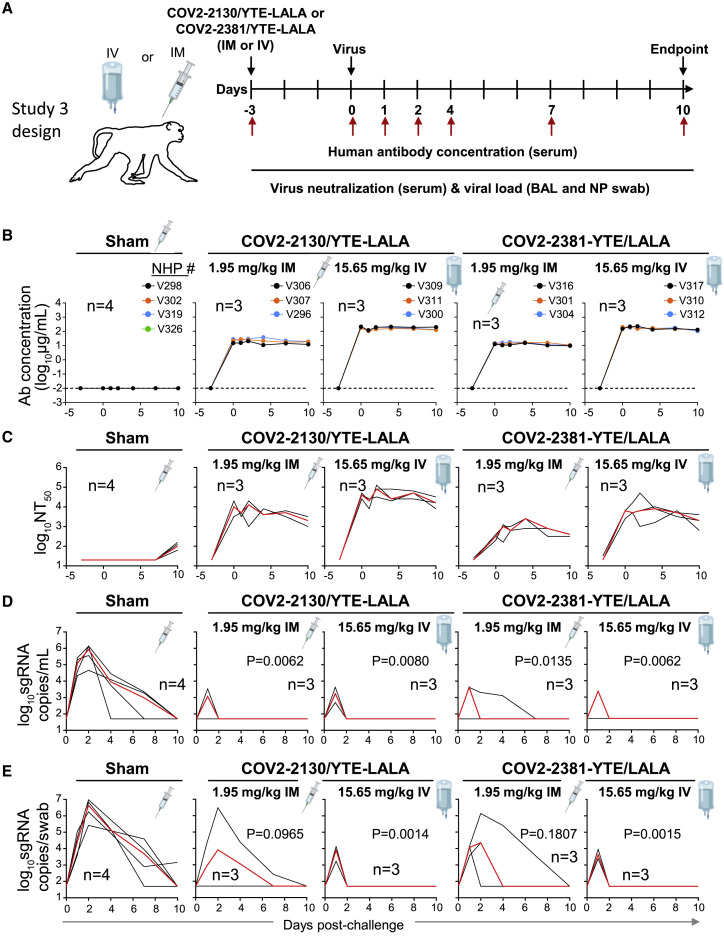
Figure 3.
Pharmacokinetics, neutralizing titers, and prophylactic efficacy of individual mAbs of the combination in SARS-CoV-2-challenged NHPs
(A) Schema of study design. Individual mAbs COV2-2130/YTE-LALA or COV2-2381/YTE-LALA (n = 3 NHPs per group) were administered to cynomolgus macaques (day −3) at different doses (1.95 or 15.65 mg/kg) and routes (IM or IV), as indicated. One group of NHPs was left untreated (sham; n = 4) to serve as controls. Animals in all groups were challenged with SARS-CoV-2 by the intranasal and intratracheal routes on day 0.
(B) Human antibody concentration was assessed by ELISA in serum at indicated time points after indicated mAb administration and viral challenge.
(C) Total neutralizing antibody titers were assessed in serum at indicated time points using a pseudovirus neutralization assay. Each black curve shows the measurements from an individual animal, with red lines indicating the median values of measurements for animals within each treatment group.
(C) sgRNA levels were assessed after viral challenge at various time points in BAL samples using qRT-PCR.
(D) sgRNA levels were assessed after viral challenge at various time points in NP swab samples.
The red line depicts the median levels of sgRNA in each group. Each black curve shows an individual animal’s measurements, with red lines indicating the median values of measurements for animals within each treatment group. Neutralization assay limit of detection = 50 copies/mL or 50 copies/swab. For statistical analysis, refer to STAR Methods section.