FIG. 1.
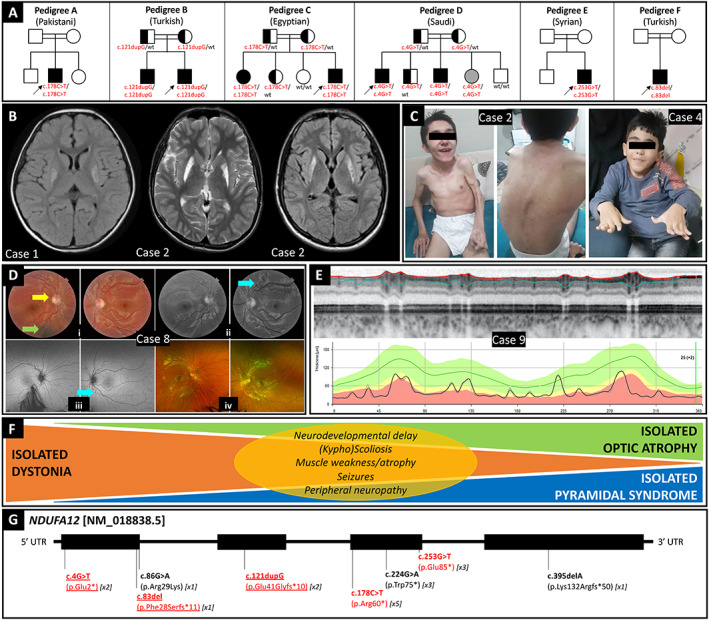
Overview of NDUFA12‐associated phenotype–genotype correlations. (A) Family trees of nine new cases herein reported and their ethnicity. Arrows identify probands. Symbols filled in with black and gray indicate homozygotes for the mutant allele who are symptomatic and asymptomatic, respectively. Half‐filled symbols represent asymptomatic heterozygous carriers of the mutant allele; wt = wild type. (B) Brain MRI of Case 1 (left, T2‐FLAIR sequence) showing hyperintense signal of the bilateral lenticular nucleus, and Case 2 (middle, T2 sequence; right, T2‐FLAIR sequence). (C) Video frames of Case 2, highlighting dystonic‐pyramidal features, kyphoscoliosis, and generalized muscle atrophy, and Case 4, showing dystonic involvement of hands and trunk as well as kyphoscoliosis. (D) Case 8: (i) Color fundus oculi showing pale optic discs and decentralized excavation with narrow temporal rim on the left eye (yellow arrow); arterial tortuosity is seen in both eyes and a choroidal nevus can be found on the right eye (green arrow). (ii) Red free photos that highlight the arteriolar tortuosity (light blue arrow). (iii) Panoramic fundus oculi picture depicts no retinal abnormalities. (iv) Normal fundus oculi autofluorescence in both eyes. (E) Case 9: Optical coherence tomography (OCT) showed markedly reduced thickness of the peripapillary nerve fiber layer. (F) Schematic of the wide phenotypic spectrum associated with biallelic loss‐of‐function variants in NDUFA12, including dystonia, pyramidal signs, and optic atrophy, either isolated or in different combinations, and additional less prevalent features (yellow oval). (G) Schematic of the NDUFA12 gene with variants hitherto reported, including those reported in the present case series (highlighted in red; novel variants also underlined). The number of symptomatic subjects carrying the variant reported so far is indicated in squared brackets.
