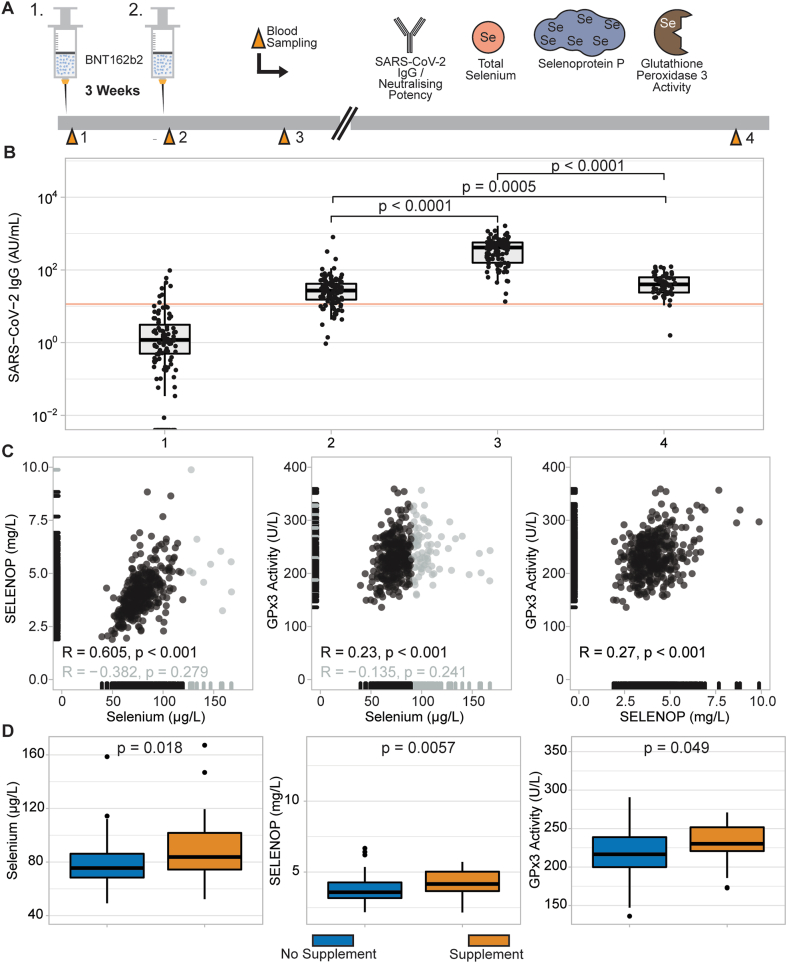
Fig. 1.
Study design, IgG response, and selenium status of the cohort. (A) Participants received two doses of BNT162b2 vaccine within a three week-interval. Blood sampling was conducted at first and second dose, three weeks and 21 weeks after the second dose. In serum samples, SARS-CoV-2 IgG titres, neutralising potency of antibodies, total Se, SELENOP, and activity of GPx3 were measured. (B) Most IgG titres were below the cut-off of seropositivity (11.5 AU/mL, red line) at time point of first sampling, except for eight participants. All volunteers reached seropositivity three weeks after the second vaccination. A waning of the IgG titres was observed at the last sampling time-point, however the titres were still significantly higher than baseline. A similar pattern for neutralising potency of the antibodies was observed (Fig. S1). (C) Serum Se and SELENOP correlated tightly below the threshold of 120 μg/L (black dots) (R = 0.605, p < 0.001), while the two parameters showed no significant correlation above 120 μg/L (gray dots). Serum Se and GPx3 activity correlated well below the threshold for GPx3 saturation (90 μg/L, black dots; R = 0.23, p < 0.001), but not above (gray dots). SELENOP and GPx3 activity correlated in the whole cohort (R = 0.27, p < 0.001). (D) Study participants who reported recent supplemental Se intake had higher levels of all three Se status biomarkers at baseline. Two-sided Wilcoxon-Rank-sum test was applied to detect differences between the two groups.