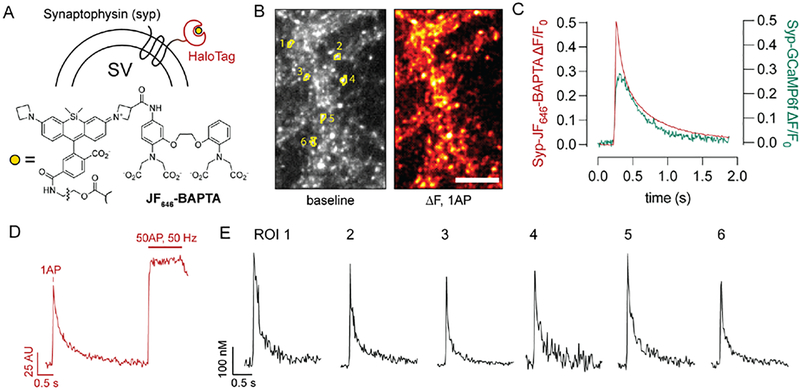
Fig. 1. A chemogenetic sensor for presynaptic Ca2+.
(A) Ca2+ sensor scheme. The HaloTag protein was targeted to nerve terminals by expression as a synaptophysin fusion construct. JF646-BAPTA bearing a HaloTag chloroalkane ligand (Deo et al., 2019) was added to the bath in AM ester form and allowed to undergo fluorogenic binding to Syp-HaloTag. (B) The reaction yields Syp-HaloTag-JF646-BAPTA, a Ca2+ sensor with bright resting fluorescence that matches the expected punctate distribution of synaptophysin labeling and readily reports presynaptic Ca2+ fluxes from single action potentials. Scale bar, 10 μm. Numbered ROIs correspond to traces shown in panels (D-E). (C) Comparison of exemplary single-stimulus, full field-of-view responses between Syp-HaloTag-JF646-BAPTA and Syp-GCaMP6f. The chemogenetic HaloTag-based approach demonstrates a substantial improvement in temporal fidelity. (D) A high-frequency stimulus train was used to obtain maximal fluorescence values for the sensor, which allows for calculation of [Ca]i (see Methods). The trace shown depicts the average fluorescence for the six labeled ROIs in panels (B) and (E). (E) Exemplary single-bouton, single-stimulus [Ca2+]i traces. Among the boutons shown here, the baseline [Ca2+]i was 102 ± 62 (s.d.) nM.