Figure 3.
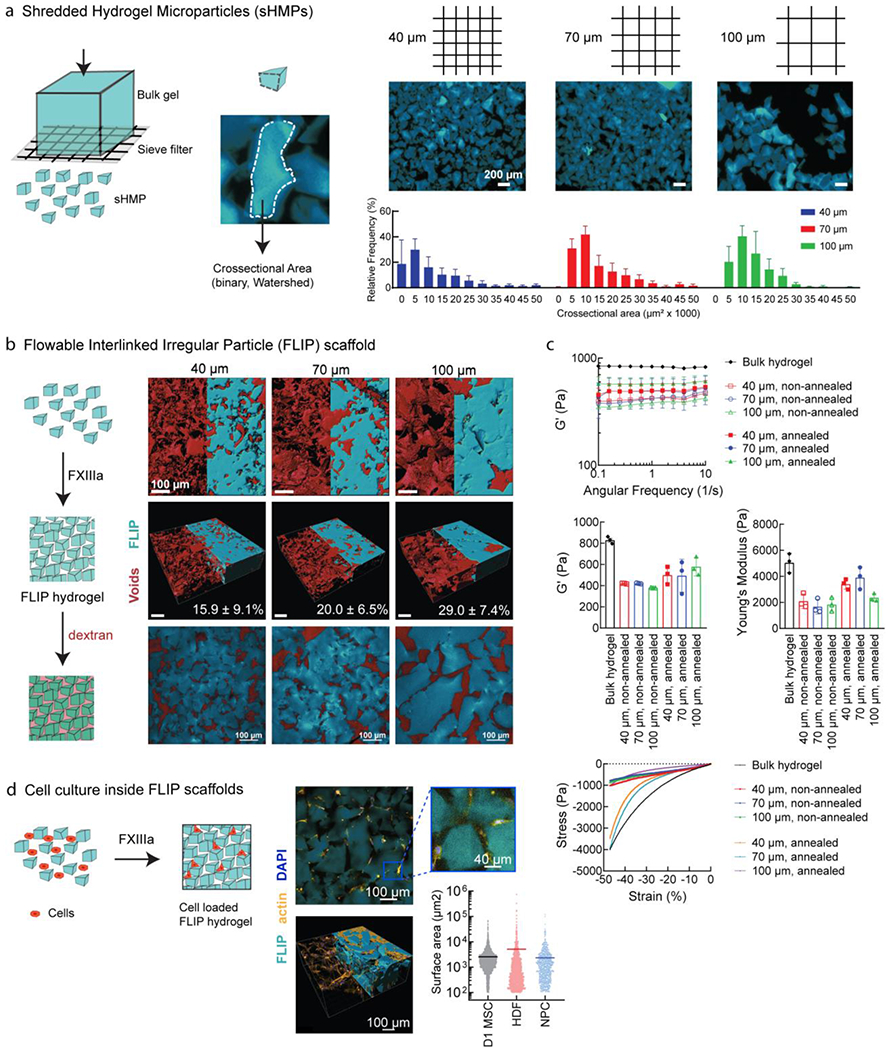
FLIP scaffold formation, mechanical properties, and 3D cell culture. (a) Formation of sHMP (shredded hydrogel microparticles) by centrifuging bulk gel disks on a cell sieve at a range of sizes (40 μm, 70 μm, and 100 μm sieve size). The resulting irregular sHMP distribution was quantified based on surface area from confocal microscopy to generate distributions. (b) Annealed FLIP scaffolds from different sieve sizes (blue), with dextran imaging of the interstitial void space (red) to quantify porosity, n = 3. Renderings performed from confocal microscopy images in IMARIS, with half corresponding to maximum intensity projection, and half from model volume filling. The third figure corresponds to the median z-slice. (c) FLIP scaffold mechanical properties. Rheology of FLIP scaffolds from 40, 70, and 100 μm sieving, with and without annealing chemistry (lack of Q/K peptides for FXIII-mediated annealing), as compared to standard bulk nanoporous HA-AC hydrogels. Frequency sweeps were performed for the stiffness (G’) while compression testing was performed for the Young’s modulus and stress-strain profiles. Sample size n=3, with error bars corresponding to S.D. (d) Cells culture is supported without loss of viability. Top-down view of actin stained cells for spreading within the FLIP scaffold, imaged half-way into the gel following seeding on top following annealing. IMARIS rendering of confocal images across a z-stack array to observe cell infiltration and spreading within the annealed scaffold. Images shown correspond to HDF culture, but data is presented as well for D1 MSCs and primary neural progenitor cells (NPC), with the horizontal bar corresponding to the mean surface area spreading and data points for each cell measured, as determined by IMARIS.
