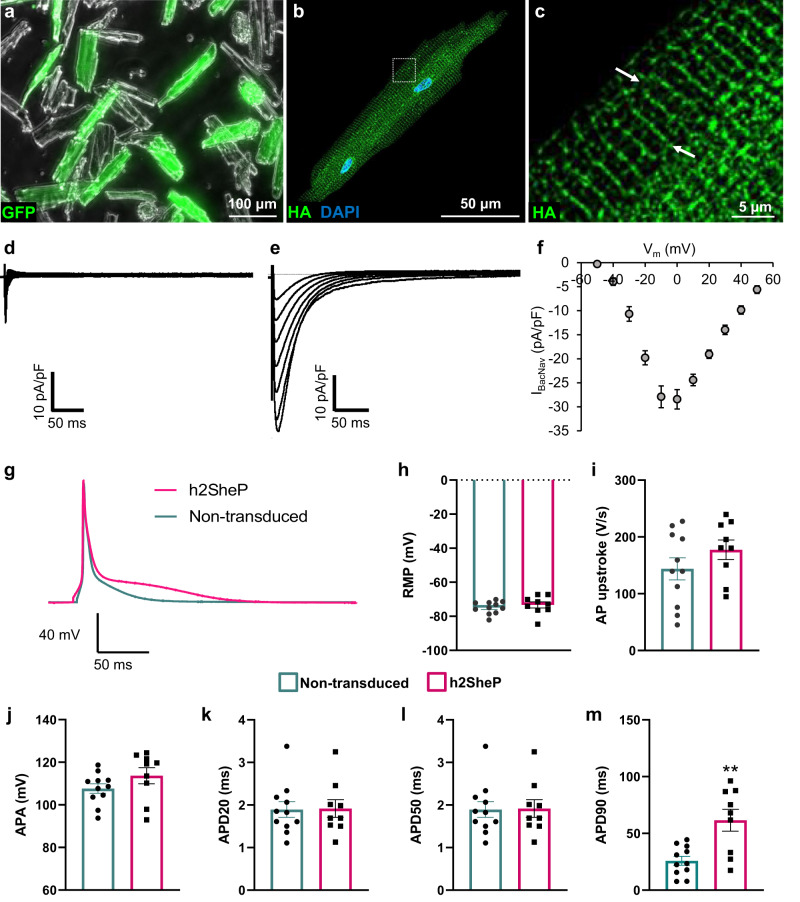
Fig. 8. Intravenous AAV-mediated delivery of BacNav yields expression of functional channels in mouse ventricular myocytes.
a–c Representative images of dissociated cardiomyocytes (CMs) (a) from mouse ventricles four weeks after tail-vein injection with 1 × 1012 vg of AAV9-MHCK7-h2SheP-HA-2A-GFP showing expression of h2SheP-HA channels at T tubules (b, c, examples shown with white arrows). d, e Representative sodium current traces in response to voltage steps from −80 mV (holding potential) to test potentials from −50 to 50 mV recorded from nontransduced (d) and transduced, h2SheP-expressing (e) mouse ventricular myocytes four weeks after tail-vein injection of 2 × 1012 vg of AAV9-CAG-h2SheP-2A-GFP. f Corresponding peak INa–V curve for CMs transduced with h2SheP virus (n = 5). Patch-clamp recordings in d–f were performed in the presence of 50 μM TTX. g–m Representative action potential (AP) traces recorded from nontransduced and transduced h2SheP-expressing mouse ventricular myocytes six weeks after tail-vein injection with 1 × 1012 vg of AAV9-MHCK7-h2SheP-HA-2A-GFP (g) and corresponding resting membrane potential (RMP, h), maximum upstroke velocity (AP upstroke, i), AP amplitude (APA, j), and durations (APD20, k; APD50, l; APD90, m, **P = 0.0017). n = 9 for nontransduced and n = 11 for transduced CMs. All patch-clamp recordings were performed at 25 °C. Error bars indicate s.e.m; statistical significance in m was determined by unpaired two-tailed t-test. Source data are provided as a Source Data file.