Table 1.
Screening an initial panel of EREDs for cyclization.
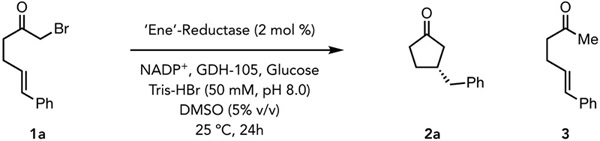
| ||||
|---|---|---|---|---|
| Entry | ‘Ene’-Reductase | Yielda (%) of 2a | erb | Yield (%) of 3 |
| 1 | OPR1 | 31 | 80:20 | 9 |
| 2 | NCR | 20 | 79:21 | 19 |
| 3 | PETNr | 66 | 66:34 | 19 |
| 4 | ERED-30c | 94 | 65:35 | 6 |
| 5 | No enzyme | 0 | n.d.d | 0 |
| 6 | No NADPH regeneration | 0 | n.d. | 0 |
Reaction conditions: α-bromoketone (1 mg, 0.004 mmol), GDH-105 (0.5 mg), NADP+ (0.5 mg), glucose (5 mg) and purified ‘ene’-reductases (2 mol%) in 50 mM Tris-HBr buffer pH 8.0, with 5% DMSO (v/v) as cosolvent, final total volume is 500 μL. Reaction mixtures were shaken under anaerobic conditions at room temperature for 24 h.
Yield determined via LCMS relative to an internal standard (TBB).
Enantiomeric ratio (er) determined by HPLC on a chiral stationary phase.
ERED-30 (1 mol%) was screened from a genetically diverse EREDs library from Prozomix.
n.d., not determined.
