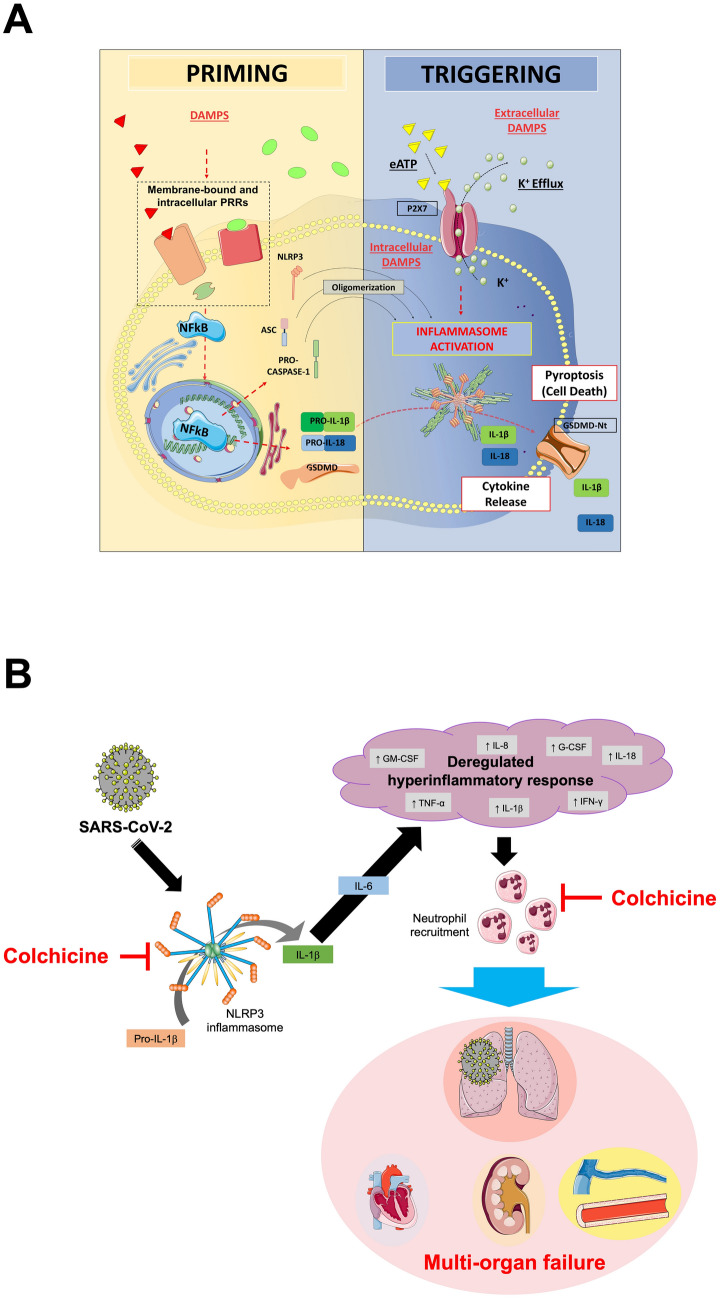
Fig. 2.
NLRP3 inflammasome formation and therapeutic implications of colchicine in COVID-19. Panel A. The formation of the NLRP3 inflammasome is a finely tuned process, that in most cases depends on two parallel pathways, i.e. priming and triggering. The priming includes signals that regulate the expression/degradation of inflammasome components (NLRP3, ASC, and caspase-1) and cytokines (IL-1β and IL-18). DAMPs activate PRRs (i.e. toll-like receptors, IL-1 receptor) leading to the translocation of the NF-kB into the nucleus. This is responsible for gene transcription of a wealth of proinflammatory genes—including inflammasome components. Except for monocytes, priming signaling by itself is not sufficient to prompt NLRP3 inflammasome activation. The translation of all inflammasome proteins is essential for the formation of the inflammasome, but does not coincide with its activation. Indeed, extra-cellular ATP or intracellular DAMPs trigger NLRP3 activation through diverse mechanisms involving the potassium efflux, namely inflammasome triggering. Once active, NLRP3 oligomerizes into a platform for recruitment of ASC and pro-caspase-1. At this stage, the activation of caspase-1 mediates the cleavage of pro-IL-1β, pro-IL-18, and gasdermin D (GSDMD). The oligomerization of the N-terminal fragment of GSDMD into a plasma membrane pore allows for the secretion of active IL-1β and IL-18 that sustain further autocrine, paracrine, and endocrine amplification of the immune response. Caspase-1 and GSDMD mediate also a form of regulated cell death known as pyroptosis. Panel B. SARS-CoV-2 induces the expression of the NLRP3 inflammasome. Following its oligomerization, NLRP3 inflammasome processes pro-inflammatory cytokines, such as IL-1β and IL-18. The release of active IL-1β triggers the production of IL-6 and leads to a dysregulated hyperinflammatory response, that is responsible for immune cell recruitment, especially neutrophils and macrophages. These events lead to organ failure, primarily the lungs, but can progress to multiorgan failure, often fatal. The figure in Panel A has been reproduced with permission from “NLRP3 Inflammasome in Acute Myocardial Infarction” by Mauro et al. [48]. The figure in Panel B has been partially created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com. DAMPs damage-associated molecular patterns. eATP extra-cellular ATP. G-CSF granulocyte colony-stimulating factor. GM-CSF granulocyte–macrophage colony-stimulating factor. GSDMD gasdermin D. GSDMD-Nt N-terminal fragment of gasdermin D. IFN interferon. IL interleukin. K+ potassium. NF-kB nuclear factor kappa-light-chain-enhancer of activated B cell. NLRP3 NACHT, leucine-rich repeat, and pyrin domain-containing protein 3. PRR pattern recognition receptor. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2. TNF tumor necrosis factor.