Figure 6.
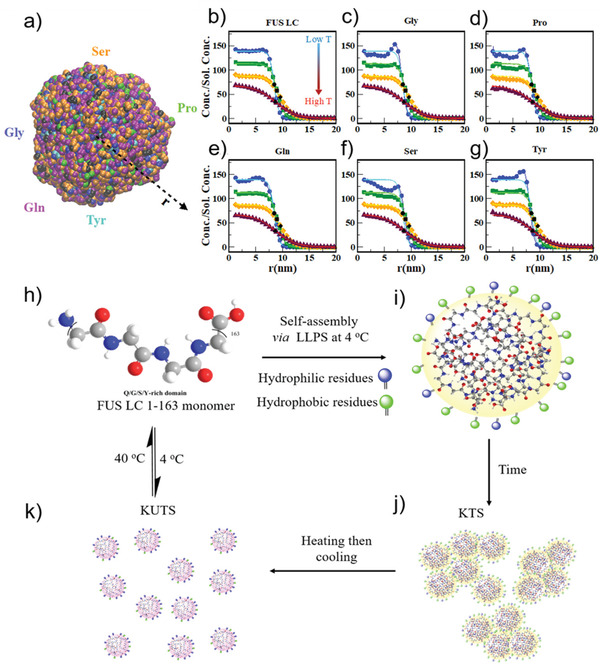
Simulations showing temperature‐dependent changes in amino acid distribution in FUS LC condensates and proposed mechanism of formation of kinetically trapped and untrapped FUS LC condensates. a) FUS LC condensate snapshot from a CG‐MD simulation of 100 chains at 100 K. CG beads are colored according to specific amino acid types (Ser, Pro, Tyr, Gln, and Gly) while the beads representing other amino acids are colored black. Concentration profiles of b) FUS LC and individual amino acids c) Gly, d) Pro, e) Gln, f) Ser, and g) Tyr at 100 K (blue, circle markers), 150 K (green, square markers), 200 K (orange, diamond markers), 230 K (maroon, triangle markers) simulation temperatures are normalized by their solution concentrations in the simulation. The solid lines in (b–g) are fits of the normalized concentration profile to a sigmoidal function and the black markers represent the inflection points for the fits to the concentration profiles with matching sybmols. h) Condensation of QGSY‐rich FUS LC (residues 1–163) via cold formation leads to i) slightly more hydrophobic residues (green circles) on the interface and j) formation of KTS condensates that show arrested coalescence. k) Annealing of KTS allows protein reconfiguration to permit additional hydrophilic residues (blue circles) to inhabit the interface and changes the bulk protein network density in KUTS condensates.
