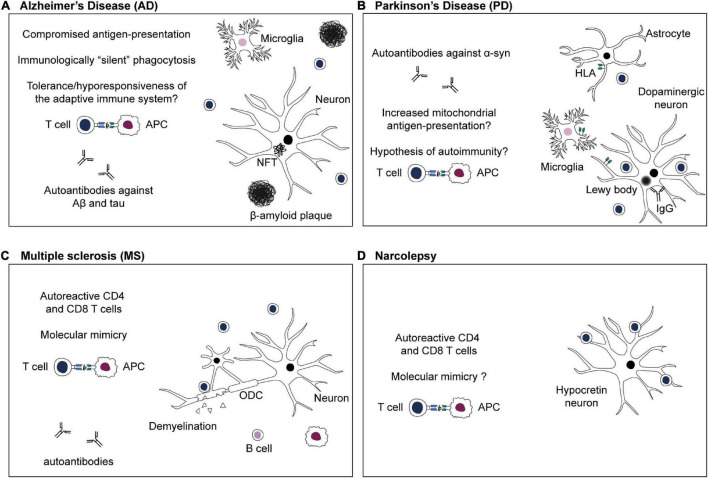
FIGURE 2.
Adaptive immune system in CNS disorders. (A) Alzheimer’s disease: the antigenic targets of infiltrating T cells in the brain during β-amyloidosis and tau pathology are not known yet. Efficient phagocytosis and antigen presentation are compromised in AD (Gu et al., 2016; Gericke et al., 2020). Phagocytosis of Aβ aggregates might happen in an immunologically “silent” manner, or, chronic exposure to Aβ-derived self-peptides and tolerance mechanisms might lead to a hyporesponsiveness of the adaptive immune system (Monsonego et al., 2001; Ferretti et al., 2016). (B) Parkinson’s disease: targeted antigen-specific T cell infiltration in the brain of PD patients is thought to be due to expression of HLA class II or class I by microglia, astrocytes, and dopaminergic neurons, respectively (Imamura et al., 2003; Cebrián et al., 2014; Rostami et al., 2020). Moreover, defects in PD-associated proteins PINK1 and parkin might increase presentation of mitochondrial antigens, leading to autoimmunity (Matheoud et al., 2016). (C) Multiple sclerosis: numerous studies emphasize the crucial role of autoreactive CD4 and CD8 T cells in MS (Dendrou et al., 2015). Autoreactive T cells infiltrate the CNS and cause demyelination as well as oligodendrocytes (ODC) and neuroaxonal damage (Dendrou et al., 2015). Autoreactive T cells might be activated in the periphery via recognition of viral antigens that are similar to a self-peptide via molecular mimicry (Sospedra and Martin, 2005; Dendrou et al., 2015). (D) Narcolepsy: autoreactive CD4 and CD8 T cells target antigens of hypocretin neurons and are thought to be responsible for neuronal damage (Latorre et al., 2018; Luo et al., 2018). The existence of T cells cross-reacting to influenza epitopes and hypocretin peptides, and the role of molecular mimicry in narcolepsy are still a subject of debate (Latorre et al., 2018: Luo et al., 2018).