Abstract
There have been few reports on the treatment of central nervous system (CNS) acute myeloid leukemia (AML) relapse. This case study demonstrates that bevacizumab may be a viable treatment option when combined with IT chemotherapy as maintenance therapy for those with CNS leukemia.
Keywords: AML, anti‐VEGF therapy, bevacizumab, central nervous system (CNS)
Bevacizumab in combination with intrathecal chemotherapy is an effective therapy for acute leukemia patients with central nervous system involvement.

1. INTRODUCTION
In contrast to acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML) has a much lower frequency of central nervous system (CNS) involvement. CNS involvement in leukemia is found in about 5–10% of adult ALL patients at diagnosis, while its involvement in AML patients results in a prevalence of 0.6%. 1 , 2 , 3 CNS is one of the most common extramedullary involvement sites in patients with AML. 4 CNS involvement is a serious complication in acute leukemia management, especially in AML after allogeneic hematopoietic stem cell transplantation (allo‐HSCT). A nested case‐control study showed that the 3‐year overall survival (OS) rate for AML patients who experienced CNS relapse after allo‐HSCT was 60.3%, significantly lower than that for patients without CNS relapse after allo‐HSCT (80.5%, p = 0.003), indicating CNS relapse after allo‐HSCT in AML patient is an adverse prognostic event. 5
After the diagnosis of CNS leukemia relapse, several treatment options such as craniospinal irradiation, intrathecal (IT) chemotherapy with methotrexate (MTX) and cytarabine, or systemic chemotherapy are widely adopted. There is no evidence in adults with CNS involvement of AML or ALL that any specific drug or combination treatment is superior, and there is a persistent concern that residual leukemic cells left after inadequate CNS treatment could cause CNS relapse. A study showed that 51 ALL children with CNS involvement had clearance of blasts from the cerebrospinal fluid (CSF) by cranial irradiation and IT chemotherapy, but only 65% of patients remained in complete remission (CR) 1 year after CNS remission with IT chemotherapy as the sole maintenance therapy, highlighting that a novel maintenance therapy to prolong the remission duration needs to be investigated. 6
Recent studies suggested an important role for vascular endothelial growth factor (VEGF) in leukemia and CNS leukemia pathogenesis. The level of VEGF in the CSF was significantly higher in patients with CNS leukemia than non‐CNS leukemia and controls. 7 Furthermore, the expressions of VEGF and VEGFR‐2 in bone marrow were significantly higher in AML patients than in controls. (p = 0.01). 8
Bevacizumab, the first angiogenesis inhibitor that targets VEGF may have an effect in acute leukemia patients, especially those with CNS involvement. The present study reports a valid experience that used bevacizumab combined with IT chemotherapy as maintenance therapy to treat CNS AML relapse after craniospinal irradiation in a 21‐year‐old allo‐HSCT female patient.
2. CASE HISTORY
A 21‐year‐old woman was diagnosed with AML with the presence of myeloblasts of 36% in peripheral blood, and a subsequent bone marrow (BM) aspiration revealed 60.5% myeloblasts. An abnormal karyotype (46, XX, inv(16)(p13q22)[10]) was identified in the bone marrow samples using a cytogenetic chromosome test. Moreover, genetic testing revealed a FLT3‐ITD–positive mutation and infusion gene of CBFβ‐MYH11. After IA (3+7) induction chemotherapy, the patient obtained complete remission (CR). She underwent consolidation chemotherapy with HiDAC (cytarabine 2 g/m2 3300 mg q12h, d1‐3) for 2 cycles. Then, in order to further lower the level of CBFβ‐MYH11, a CLAG regimen (cladribine 5 mg/m2 d1‐5, cytarabine1 g/m2 d1‐5, G‐CSF 5 μg/kg d1‐5) was given. In consideration of the persistent presence of CBFβ‐MYH11 fusion gene, she received the allo‐HSCT, with her younger brother, who has matched human leukocyte antigen (HLA), used as the donor. She developed chronic graft versus host disease (GVHD) of intestine and liver after allo‐HSCT, methylprednisolone and cyclosporin were used to control GVHD. CMV viremia, peripheral neuropathy, and joint pain occurred in succession, the aforementioned conditions above turning better after corresponding therapy.
Two years after allo‐HSCT, 18.5% myeloblasts were identified in the bone marrow, confirming the relapse of the disease. She received subcutaneous injection with azacitidine (75 mg/m2, days 1–7), and sorafenib treatment orally (400 mg twice daily) for 1 month, but it failed to achieve CR. Then, she was started on HAG (homoharringtonine 2 mg intravenous infusion qd on d1‐8, cytarabine 10 mg/m2 subcutaneous injection q12h on d1‐14, G‐CSF 300 μg subcutaneous injection qd on d1‐14) regimen and achieved CR. However, 4 months later, epilepsy occurred. Head CT showed a mass with abnormal density in the right temporal and parietal lobes, a ring enhancing density at the edge (Figure 1). Flow cytometry of CSF analyzed 2836 cells, revealed 660 abnormal myeloid blasts to be positive for CD34, CD117 expression, accounting for 23.3%. The cells expressed CD33, HLA‐DR, but negative for CD13, CD7, and CD10 (Figure 2). Taking into account of her symptoms and examination reports, CNS AML relapse was established.
FIGURE 1.

Head computed tomography demonstrating a mass with abnormal density in the right temporal and parietal lobes. (A), CT at diagnosis. (B), CT after IT chemotherapy solely. (C), CT after craniospinal irradiation. (D), CT after bevacizumab combined therapy
FIGURE 2.
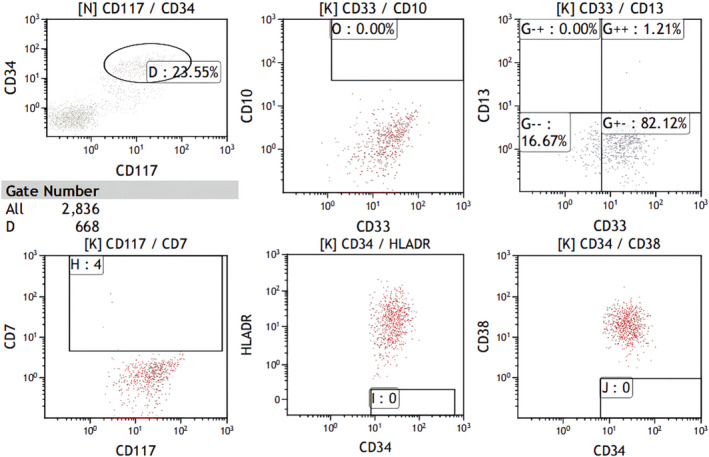
Flow cytometric analysis of CSF at CNS AML relapse
Subsequently, furosemide and mannitol were used to reduce cranial pressure and levetiracetam was used to control the epilepsy caused by CNS AML. She also received IT chemotherapy (cytarabine 40 mg, dexamethasone 5 mg) once a week to control CNS AML for seven cycles. Nevertheless, the headache aggravated and the lesion became larger, indicating that the IT chemotherapy alone was ineffective. Then, she received craniospinal irradiation therapy at a dose of 3600 cGy in 18 fractions targeted at lesion, a cranial irradiation at a dose of 3000 cGy in 15 fractions, a spinal irradiation at a dose of 2400 cGy in 12 fractions, which was administered over a 1‐month period. The CT revealed obvious shrink of lesion but she just attained the criteria of no change (NC) according to the criteria of treatment response in solid tumor recommended by WHO. 9 Blast cells were not detected in CSF after cytological examination. In the following days, IT chemotherapy of cytarabine 40 mg, dexamethasone 5 mg were given every 2 months, in combination with bevacizumab 300 mg (7.5 mg/kg) every 3 weeks for five cycles, the condition was stable and the CT revealed partial remission (PR) of lesion according to the criteria of treatment response in solid tumor recommended by WHO. The patient has remained in continuous complete remission in bone marrow and PR in CNS for almost 1 year after initiation of bevacizumab combination treatment at present (Figure 3).
FIGURE 3.
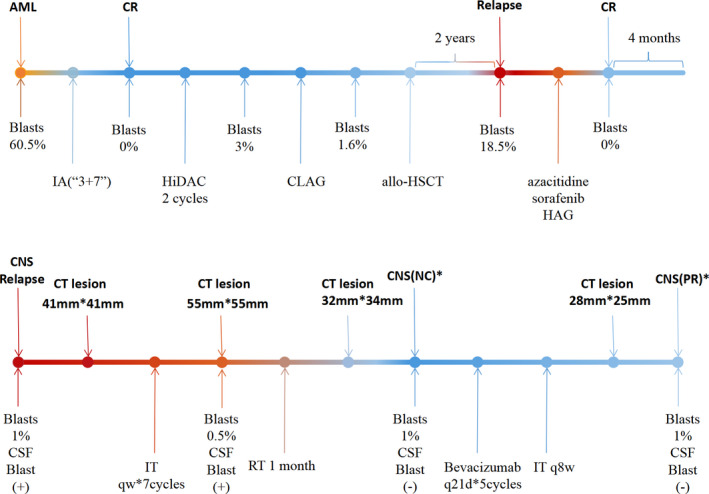
Treatment process of the patient CR, complete remission; CNS NC*, a 50% decrease in total tumor size cannot be established nor has a 25% increase in the size of one or more measurable lesions been demonstrated; CNS PR*, single lesion, greater than or equal to 50% decrease in tumor area (multiplication of longest diameter by the greatest perpendicular diameter); IA, cytarabine 100 mg/m2 continuous infusion d1‐7, idarubicin 10 mg/m2 d1‐3; HiDAC, cytarabine 2 g/m2 every 12 h on d1‐3; CLAG, cladribine 5 mg/m2 d1‐5, cytarabine1 g/m2 d1‐5, G‐CSF 5 μg/kg d1‐5; HAG, homoharringtonine 2 mg Intravenous infusion qd on d1‐8, cytarabine 10 mg/m2 subcutaneous injection q12h on d1‐14, G‐CSF 300 μg subcutaneous injection qd on d1‐14; IT, intrathecal (cytarabine 40 mg, dexamethasone 5 mg); RT, radiation therapy; allo‐HSCT, allogeneic hematopoietic stem cell transplantation
3. DISCUSSION
Acute leukemia with CNS involvement is a serious complication in clinical practice. It remains unclear how leukemic cells penetrate into CNS. A previous study suggests that up‐regulation of VEGF‐A may have a correlation with BBB breakdown, which implicates a potential role VEGF plays in the pathogenesis of CNS leukemia. 10 Tang et al reported that the levels of VEGF‐A in CSF in CNS leukemia including ALL and AML patients were elevated compared with non‐CNSL patients and controls (p < 0.05). 7 They also found that the VEGF‐A level in CSF was higher than that in Serum in the CNS leukemia groups only (p = 0.004). Besides, Münch et al found that gene coding for VEGF and VEGF expression was highly upregulated in CNS‐derived ALL cells. 11 The reasons for VEGF accumulation in CSF could be due to secretion by the leukemia cells infiltrated into CNS.
Bevacizumab, is a monoclonal antibody that can bind and inactivate all isoforms of VEGF to inhibit angiogenesis and tumor growth and proliferation. 12 , 13 Interestingly, in the presence of bevacizumab, the growth, proliferation, and survival of ALL cells are not affected, while the migration ability is decreased when cultured with bevacizumab. However, a similar impact from bevacizumab on AML cells has not been illustrated. However, Katoh et al showed that resistance to radiotherapy by human megakaryocytic leukemia cell lines CMK86 could be improved by VEGF in an autocrine or paracrine manner. 14 Avramis et al also reported that VEGF could improve the resistance ability of human leukemia T‐cell lines to taxotere and vincristine, while inhibiting VEGF could improve the sensitivity of these cell lines to chemotherapy. 15 These in vitro studies suggested that anti‐VEGF therapy may have synergistic antitumor effect with irradiation therapy and chemotherapy. Furthermore, clinical trials investigating the feasibility and efficacy of VEGF inhibition strategy in a variety of malignancies are ongoing. However, few studies have incorporated bevacizumab in the regimen to treat acute leukemia. Judith et al combined bevacizumab with Sequential 1‐β‐D‐Arabinofuranosylcytosine and mitoxantrone therapy, which showed favorable outcome in relapse/refractory (R/R) AML patients: the CR rate was 33%, and 1‐year event‐free survival (EFS) was 64%. 16 Zahiragic et al demonstrated that the usage of single agent bevacizumab at a dose of 10 mg/kg body weight which was administered as intravenous infusion in the R/R AML patients who are not qualified for further intensive cytotoxic chemotherapy lead to the down regulation of VEGF in the bone marrow. 17 Although it didn't display any significant antileukemic activity, and none of the patients fulfilled the criteria of a partial response, it was well tolerated with a favorable safety profile. No hematological related adverse events were observed in this cohort. Although, severe adverse events (SAEs) were reported to be higher in the chemotherapy plus bevacizumab arm compared with the chemotherapy arm in the treatment of AML patients (p = 0.043), which may be due to the elder patients constitutions in the cohort. 18
In our case study, the 21‐year‐old woman with high risk of CNS AML involvement related to FLT3 ITD mutation eventually relapsed in CNS 2 years after allo‐HSCT. 3 , 19 The craniospinal irradiation therapy apparently shrunk the lesion with a high dose of 3600 cGY in the target lesion, divided into 18 applications to complete the treatment. Simultaneously, we also conducted extension irradiation coincident with time of target irradiation at the doses of 3000 cGy and 2400 cGy. The patient's condition was stable and the lesion further shrunk to achieve PR with the combined therapy of IT chemotherapy and bevacizumab as maintenance therapy, while she had not shown response to IT chemotherapy alone in prior treatment. We had administrated bevacizumab in the treatment of CNS leukemia, and combined it with IT chemotherapy.
To our knowledge, this is the first effective case report of bevacizumab in combination with IT chemotherapy as a maintenance therapy in CNS AML relapse after craniospinal irradiation. After administration of the combination of these two treatment regimens, the patient stayed in good condition after the diagnosis of CNSL for almost 1 year to date. The treatment choice for CNS leukemia is limited, while there exists promising chimeric antigen receptor T‐cell (CAR‐T) immunotherapy for B‐ALL CNS leukemia. 20 , 21 CAR‐T therapy for AML and CNS AML still needs urgent investigation.
In conclusion, IT chemotherapy combined with bevacizumab may be a viable option for AML patient with CNS involvement. It is effective as a maintenance therapy for CNS relapse especially when the disease is controlled after craniospinal irradiation as this case illustrated. Based on the potential role played by VEGF in the pathogenesis of CNS leukemia and a series of in vitro and in vivo studies, bevacizumab may also be considered as a prophylactic drug for CNS relapse. It appears that the dose of 300 mg bevacizumab is safe for this patient without any adverse event occurring. Considering that adverse events related with bevacizumab, including up‐regulation of blood pressure and thrombosis were observed in previous reports, the application of bevacizumab to older patients needs more stringent assessment. Moreover, there is no consensus for CNS AML therapy in published reports and our case provides a new light on the treatment of CNS AML. A well‐designed, prospective study will aid further to validate the effect of these combined regimens.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
HYC performed literature review, drafted manuscript. TT involved in visualization. XDS was involved in clinical care of the patient. LB, CLW, and DPW edited the manuscript. JLL and SLX involved in supervision.
ETHICAL APPROVAL
The patient has provided written informed consent for the publication of this case report.
CONSENT
Written informed consent of the patient was obtained for publication.
ACKNOWLEDGEMENT
None.
Cao H‐Y, Tao T, Shen X‐D, et al. Efficiency of anti‐VEGF therapy in central nervous system AML relapse: A case report and literature review. Clin Case Rep. 2022;10:e05367. doi: 10.1002/ccr3.5367
Han‐Yu Cao, Tao Tao, Xiang‐Dong Shen and Lian Bai equal contribution.
Funding information
This work was supported by the grants from the National Natural Science Foundation of China (grant No. 81970138), Translational Research Grant of NCRCH (grant No. 2020ZKMB05)
Contributor Information
Jin‐Li Li, Email: ljl2049@163.com.
Sheng‐Li Xue, Email: slxue@suda.edu.cn.
DATA AVAILABILITY STATEMENT
The data presented in this study are available in this article.
REFERENCES
- 1. Abbott BL, Rubnitz JE, Tong X, et al. Clinical significance of central nervous system involvement at diagnosis of pediatric acute myeloid leukemia: a single institution's experience. Leukemia. 2003;17:2090‐2096. [DOI] [PubMed] [Google Scholar]
- 2. Larson RA. Managing CNS disease in adults with acute lymphoblastic leukemia. Leuk Lymphoma. 2018;59(1):3‐13. [DOI] [PubMed] [Google Scholar]
- 3. Alakel N, Stölzel F, Mohr B, et al. Symptomatic central nervous system involvement in adult patients with acute myeloid leukemia. Cancer Manag Res. 2017;9:97‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim JY, Im SA, Lee JH, Lee JW, Chung NG, Cho B. Extramedullary relapse of acute myeloid and lymphoid leukemia in children: a retrospective analysis. Iran J Pediatr. 2016;26:e1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Q, Zhu XL, Zhao X, et al. Prognosis and risk factors for central nervous system relapse after allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia. Ann Hematol. 2021;100:505‐516. [DOI] [PubMed] [Google Scholar]
- 6. Land VJ, Thomas PR, Boyett JM, et al. Comparison of maintenance treatment regimens for first central nervous system relapse in children with acute lymphocytic leukemia. A pediatric oncology group study. Cancer. 1985;56:81‐87. [DOI] [PubMed] [Google Scholar]
- 7. Tang YT, Jiang F, Guo L, Si MY, Jiao XY. Expression and significance of vascular endothelial growth factor A and C in leukemia central nervous system metastasis. Leuk Res. 2013;37:359‐366. [DOI] [PubMed] [Google Scholar]
- 8. Padró T, Bieker R, Ruiz S, et al. Overexpression of vascular endothelial growth factor (VEGF) and its cellular receptor KDR (VEGFR‐2) in the bone marrow of patients with acute myeloid leukemia. Leukemia. 2002;16:1302‐1310. [DOI] [PubMed] [Google Scholar]
- 9. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207‐214. [DOI] [PubMed] [Google Scholar]
- 10. Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF‐mediated disruption of endothelial CLN‐5 promotes blood‐brain barrier breakdown. Proc Natl Acad Sci USA. 2009;106:1977‐1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Münch V, Trentin L, Herzig J, et al. Central nervous system involvement in acute lymphoblastic leukemia is mediated by vascular endothelial growth factor. Blood. 2017;130:643‐654. [DOI] [PubMed] [Google Scholar]
- 12. Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671‐680. [PubMed] [Google Scholar]
- 13. Zondor SD, Medina PJ. Bevacizumab: an angiogenesis inhibitor with efficacy in colorectal and other malignancies. Ann Pharmacother. 2004;38:1258‐1264. [DOI] [PubMed] [Google Scholar]
- 14. Katoh O, Tauchi H, Kawaishi K, Kimura A, Satow Y. Expression of the vascular endothelial growth factor (VEGF) receptor gene, KDR, in hematopoietic cells and inhibitory effect of VEGF on apoptotic cell death caused by ionizing radiation. Cancer Res. 1995;55:5687‐5692. [PubMed] [Google Scholar]
- 15. Avramis IA, Kwock R, Avramis VI. Taxotere and vincristine inhibit the secretion of the angiogenesis inducing vascular endothelial growth factor (VEGF) by wild‐type and drug‐resistant human leukemia T‐cell lines. Anticancer Res. 2001;21:2281‐2286. [PubMed] [Google Scholar]
- 16. Karp JE, Gojo I, Pili R, et al. Targeting vascular endothelial growth factor for relapsed and refractory adult acute myelogenous leukemias: therapy with sequential 1‐beta‐d‐arabinofuranosylcytosine, mitoxantrone, and bevacizumab. Clin Cancer Res. 2004;10:3577‐3585. [DOI] [PubMed] [Google Scholar]
- 17. Zahiragic L, Schliemann C, Bieker R, et al. Bevacizumab reduces VEGF expression in patients with relapsed and refractory acute myeloid leukemia without clinical antileukemic activity. Leukemia. 2007;21:1310‐1312. [DOI] [PubMed] [Google Scholar]
- 18. Ossenkoppele GJ, Stussi G, Maertens J, et al. Addition of bevacizumab to chemotherapy in acute myeloid leukemia at older age: a randomized phase 2 trial of the Dutch‐Belgian Cooperative Trial Group for Hemato‐Oncology (HOVON) and the Swiss Group for Clinical Cancer Research (SAKK). Blood. 2012;120:4706‐4711. [DOI] [PubMed] [Google Scholar]
- 19. Shihadeh F, Reed V, Faderl S, et al. Cytogenetic profile of patients with acute myeloid leukemia and central nervous system disease. Cancer. 2012;118:112‐117. [DOI] [PubMed] [Google Scholar]
- 20. Lee DW, Kochenderfer JN, Stetler‐Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose‐escalation trial. Lancet. 2015;385:517‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pehlivan KC, Duncan BB, Lee DW. CAR‐T cell therapy for acute lymphoblastic leukemia: transforming the treatment of relapsed and refractory disease. Curr Hematol Malig Rep. 2018;13:396‐406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this article.


