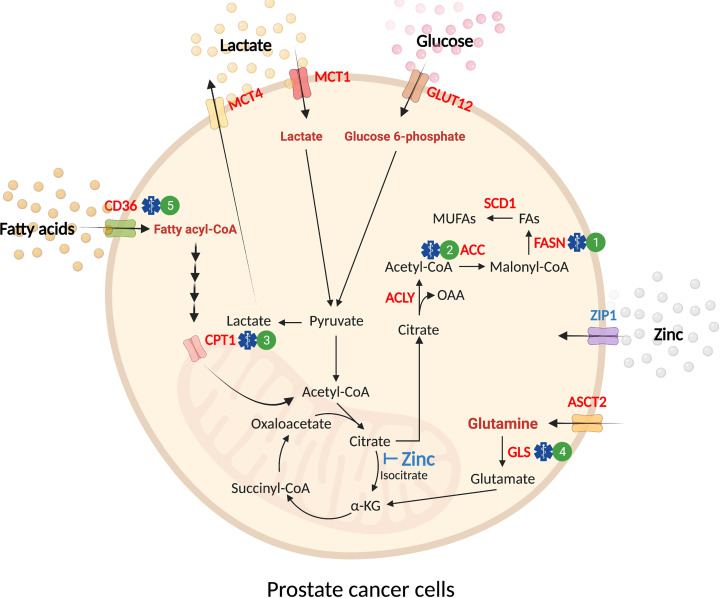
Figure 2.
Substrate utilization in prostate cancer. Normal prostate epithelial cells exhibit a glycolytic phenotype due to the inhibitory effect of mitochondrial zinc accumulation in the TCA cycle (blue text). Malignant transformation of prostate epithelial cells leads to an increase in the uptake of exogenous nutrients (glucose, glutamine, fatty acids, and lactate) and de novo synthesis of lipids (red text). These substrates are utilized for energy production in the mitochondria to accommodate increasing energy demands in malignancy. In prostate cancer, glucose uptake is mediated by GLUT12 before it is catabolized into pyruvate. While a proportion of glucose-derived pyruvate enters the TCA cycle for oxidation, a fraction of pyruvate is reduced to lactate and transported out of the cell by MCT4. The influx of extracellular lactate is mediated by MCT1. In mitochondria, the outflow of citrate to cytosol provides substrate for de novo synthesis of fatty acids (i.e. lipogenesis). ASCT2 supplies exogenous glutamine as a fuel source through deamination by glutaminase (GLS) before further conversion into ⍺-KG to feed the TCA cycle. Fatty acid uptake is mediated by fatty acid translocase (FAT)/CD36 before transport into the mitochondria by CPT1. In mitochondria, fatty acids undergo β-oxidation, producing acetyl-CoA that feeds into the TCA cycle. Pre-clinical treatments for prostate cancer are denoted by a blue symbol and corresponding number. 1) FASN (e.g. TVB-2640, IPI-9119); 2) ACC (e.g. Firsocostat, PF-05175157); 3) CPT1 (e.g. Perhexiline); 4) GLS (e.g. CB-839); 5) CD36 (e.g. agents in development). ⍺-KG, ⍺-ketoglutarate; CPT1, carnitine palmitoyltransferase 1; FAs, fatty acids; GLS, glutaminase; MCT1, monocarboxylate transporter 1; MCT4, monocarboxylate transporter 4; MUFAs, monounsaturated fatty acids; OAA, oxaloacetate.