Figure 6.
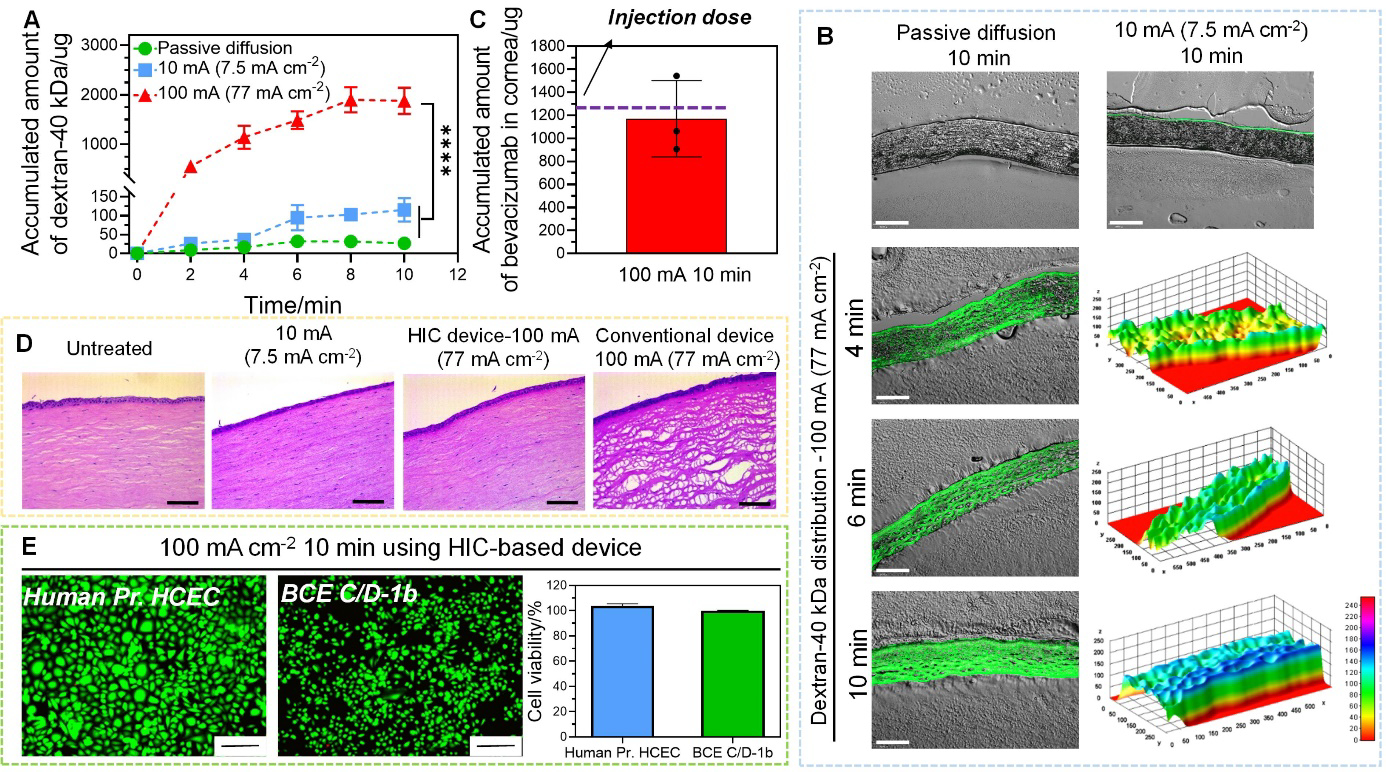
Intracorneal delivery of dextran-40 kDa and bevacizumab by high-intensity iontophoresis using HIC-based device. (A) Accumulated amount of dextran-40 kDa extracted from cornea after passive diffusion, low-intensity iontophoresis at 10 mA (7.5 mA cm−2), and high-intensity iontophoresis at 100 mA (77 mA cm−2) applied for 2 min, 4 min, 6 min, 8 min, and 10 min. (B) Fluorescent images of cryo-sectioned rabbit corneas after intracorneal delivery of dextran-40 kDa under different conditions (passive diffusion, low-intensity iontophoresis at 10 mA (7.5 mA cm−2), and high-intensity iontophoresis at 100 mA (77 mA cm−2)). The fluorescent intensity was converted to a 3D stack using surface plotting from ImageJ software for cornea samples treated with high-intensity iontophoresis (scale bar: 200 μm). (C) Accumulated amount of bevacizumab extracted from cornea after high-intensity iontophoresis at 100 mA (77 mA cm−2) applied for 10 min. (D) H&E images for histological analysis of the cornea after iontophoresis under different test conditions (no treatment, low-intensity iontophoresis at 10 mA (7.5 mA cm−2) applied by our HIC-based device for 10 min, high-intensity iontophoresis at 100 mA (77 mA cm−2) applied by our HIC-based device and a conventional iontophoresis device for 10 min) (scale bar: 25 μm). (E) The viability of corneal epithelial (Human Pr. HCEC) and endothelial cell (BCE C/D-1b) (normalized to untreated control) after treated by 100 mA (77 mA cm−2) current for 10 min by our HIC-based device (scale bar: 200 μm).
