Figure 2.
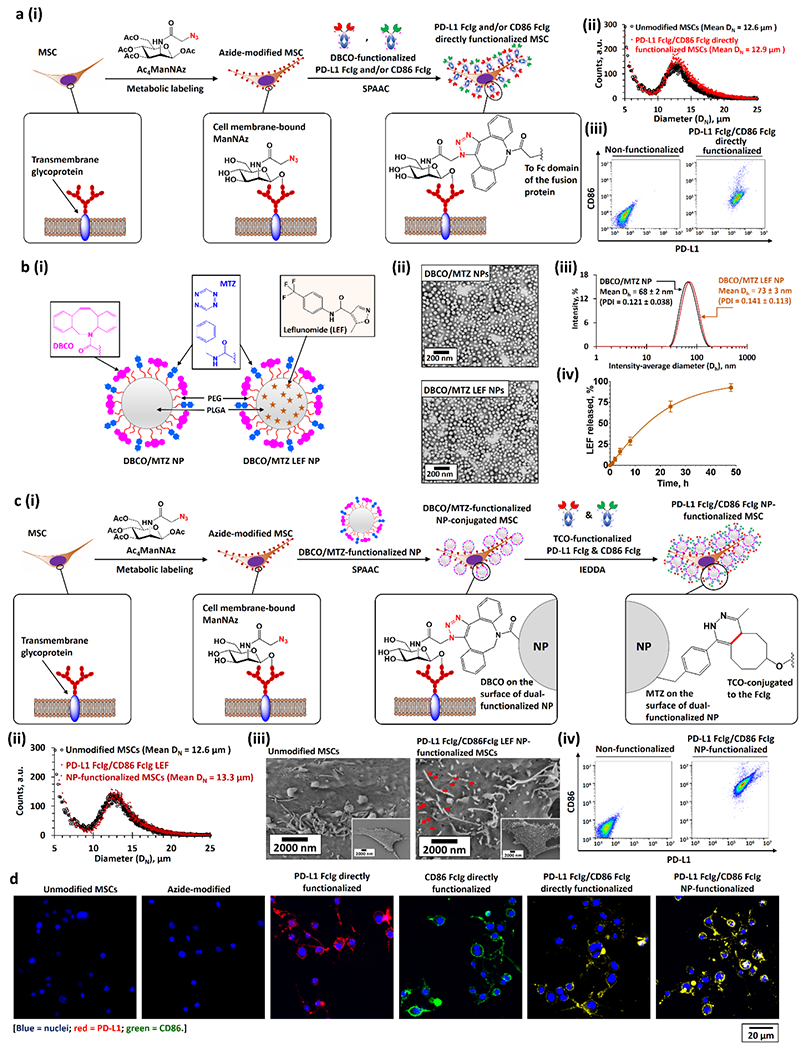
Bioengineering of PD-L1 and CD86 functionalized MSCs. a)(i) Bioengineering PD-L1 Fc-Ig and CD86 Fc-Ig directly functionalized MSCs through metabolic glycoengineering followed by SPAAC with DBCO-functionalized PD-L1 Fc-Ig and CD86 Fc-Ig. (ii, iii) Size distributions (ii), PD-L1, and CD86 expressions (iii) of unmodified and PD-L1 Fc-Ig and CD86 Fc-Ig directly functionalized MSCs. b)(i) Structures of drug-free DBCO and MTZ dual-functionalized PEG-PLGA NPs (DBCO/MTZ NPs) and LEF-encapsulated DBCO/MTZ NPs (DBCO/MTZ LEF NPs). (ii, iii) TEM images (ii), and intensity-average diameter (Dh) distributions (iii) of drug-free and LEF-encapsulated DBCO and MTZ dual-functionalized NPs. (iv) Drug-release profile of LEF-encapsulated DBCO and MTZ dual-functionalized NPs at physiological conditions in the presence of large excess of PBS. c)(i) Bioengineering of PD-L1 Fc-Ig and CD86 Fc-Ig NP-dual-functionalized MSCs. The dual-functionalized MSCs were engineered via 3 steps: first, metabolic labeling of Ac4ManNAZ gave azide-modified MSCs; second, the conjugation of DBCO/MTZ NPs (or DBCO/MTZ LEF NPs) onto the azide-modified MSCs through SPAAC at the physiological conditions; and finally, the bioconjugation of TCO-functionalized PD-L1 Fc-Ig and CD86 Fc-Ig onto the DBCO/MTZ NP-functionalized MSCs via IEDDA at the physiological conditions. (ii–iv) Size-distributions (ii), scanning electron microscopy (SEM) images (iii), and PD-L1 and CD86 expressions (iv) of unmodified and PD-L1 Fc-Ig and CD86 Fc-Ig NP-functionalized MSCs. Pseudopodia can be identified from the SME images of both unmodified and functionalized MSCs. The red arrows in the SEM images highlighted the PD-L1 FcIg/CD86 FcIg LEF NPs grafted on the surface of the MSCs. d) Representative CLSM images of different as-functionalized MSCs.
