Figure 5.
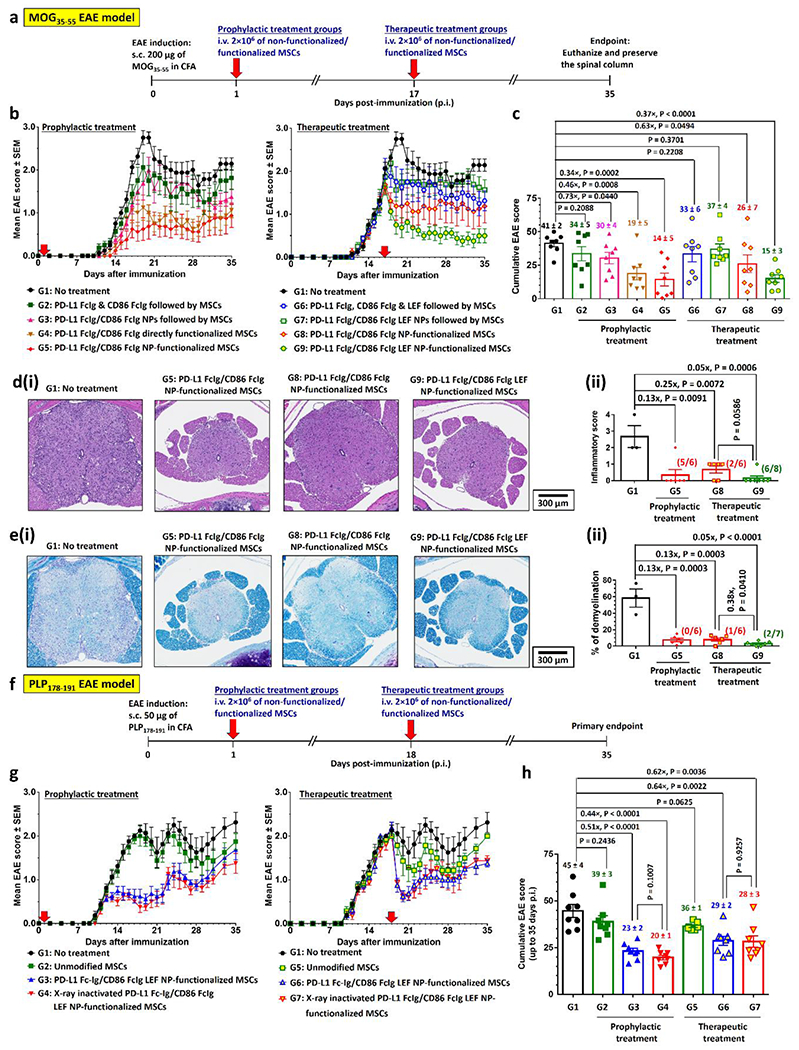
PD-L1- and CD86-conjugated NP-functionalized MSCs effectively suppress progressive chronic MOG35-55-EAE model and relapsing-remitting PLP178-191-EAE model in vivo, prophylactically, and therapeutically. a) Prophylactic and therapeutic treatment schedules with PD-L1 FcIg/CD86 FcIg NP-functionalized MSCs in C57BL/6 mice after immunization with MOG35-55 peptide. Unmodified or functionalized MSCs were i.v. administrated 1 day (prophylactic treatment) or 17 days (therapeutic treatment) p.i.. Body conditions were monitor daily until 35 days p.i. Mice were euthanized 36 or 37 days p.i., spinal columns were preserved for further histopathological studies. In control treatment groups 2, 3, 6, and 7, free or NP conjugated PD-L1 Fc-Ig, and CD86 Fc-Ig (plus unencapsulated LEF) were i.v. administrated 20 min before the non-functionalized MSCs. b) Time-dependent mean clinical scores of MOG35-55-induced EAE inflicted mice after received different prophylactic and therapeutic treatments. (n = 8 mice per group; one non-treatment group mouse was found dead 28 days p.i.) c) Cumulative EAE scores of MOG35-55-EAE inflicted mice after received different treatments. d)(i) Representative H&E-stained spinal cord sections preserved from EAE-inflicted mice after received different prophylactic and therapeutic treatments with drug-free/LEF-encapsulated PD-L1 FcIg/CD86 FcIg NP-functionalized MSCs. (ii) Quantification of spinal inflammation from the H&E-stained images of spinal cords. (n = 3 for the non-treatment group; n = 6 for the prophylactic treatment group and therapeutic treatment group treated with drug-free PD-L1 FcIg/CD86 FcIg NP-functionalized MSCs; n = 7 for the therapeutic treatment group treated with PD-L1 FcIg/CD86 FcIg LEF NP-functionalized MSCs.) e)(i) Representative LFB-stained spinal cord sections preserved from EAE-inflicted mice after received different prophylactic and therapeutic treatments with drug-free/LEF-encapsulated PD-L1 FcIg/CD86 FcIg NP-functionalized MSCs. (ii) Quantification of demyelination from the LFB-stained images of spinal cords. (n = 3 for the non-treatment group; n = 6 for the prophylactic treatment group and therapeutic treatment group treated with drug-free PD-L1 FcIg/CD86 FcIg NP-functionalized MSCs; n = 7 for the therapeutic treatment group treated with PD-L1 FcIg/CD86 FcIg LEF NP-functionalized MSCs.) f) Prophylactic and therapeutic treatment schedules with PD-L1 FcIg/CD86 FcIg NP-functionalized MSCs in C57BL/6 mice after immunization with PLP178-191 peptide. Unmodified or functionalized MSCs were i.v. administrated 1 day (prophylactic treatment) or 18 days (therapeutic treatment) p.i.. Body conditions were monitor until 35 days p.i. g) Time-dependent mean clinical scores of MOG35-55-induced EAE inflicted mice after received different prophylactic and therapeutic treatments. (n = 8 mice per group, except n = 7 for the therapeutic treatment group with unmodified MSCs.) h) Cumulative EAE scores of PLP178-191-EAE inflicted mice after received different treatments.
