Summary
Background
Patients with PD(L)-1 therapy resistant non-small cell lung cancer (NSCLC) have poor outcomes. Studies suggest radiotherapy may enhance anti-tumor immunity. Therefore, we investigated combined PD-L1 (durvalumab) and CTLA-4 inhibition (tremelimumab) alone or combined with radiation.
Methods
This multicentre, randomised, open-label phase 2 NCI Experimental Therapeutics Clinical Trials Network trial was conducted at 18 U.S. sites. Patients aged >=18 years with metastatic NSCLC, Eastern Cooperative Oncology Group performance status of 0 or 1, and progression during previous PD(L)-1 therapy were randomised (1:1:1) using a permuted block scheme, without stratification to durvalumab/tremelimumab (1500mg/75mg every 4 weeks, 4 cycles, intravenous) alone (noRT) or with low-dose (LDFRT) or hypofractionated (HFRT) radiotherapy followed by durvalumab until 52 weeks or progression. The primary endpoint was the rate of complete/partial response in patients who initiated study treatment. The trial is registered with ClinicalTrials.gov, NCT02888743, and is now complete.
Findings
90 patients were randomised and 78 patients treated between 24 August 2017 and 29 March 2019. Median follow up was 12.4 months (IQR 7.8-15.1). There were no differences in response rates between the noRT arm (3 [11.5%] of 26, 90% CI 1.2-21.8) and the LDFRT (2 [7.7%] of 26, 0-16.3, p=0.64) or HFRT (3 [11.5%] of 26, 90% CI 1.2-21.8, p=0.99) arms. The most common grade 3-4 adverse event was dyspnea (2 [8%] of 26 NoRT, 3 [11%] LDFRT, and 3 [11%] HFRT). Serious adverse events occurred in 4 (15%, NoRT), 8 (31%, LDFRT) and 3 (12%, HFRT) patients. There was 1 grade-5 respiratory failure potentially related to therapy (LDFRT arm).
Interpretation
Radiation should not be used to increase response to PD-L1/CTLA-4 inhibition in PD(L)-1-resistant NSCLC patients. However, PD-L1/CTLA-4 therapy could be a treatment option for some patients. Future studies should refine predictive biomarkers in this setting.
Funding:
US National Institutes of Health, Dana-Farber Cancer Institute
Introduction
PD(L)-1 immune checkpoint inhibitors have benefitted patients with metastatic NSCLC either alone or with chemotherapy, and are used in the first line setting.1-4 However, the majority of patients do not respond or progress on therapy.5,6 Treatment options in the setting of resistance to PD(L)-1 blockade are limited, and outcomes on standard therapy are poor. Combined PD(L)-1 / CTLA-4 inhibition demonstrated synergy in preclinical models,7 and has been approved as first-line therapy for metastatic NSCLC,8-10 but it is unclear if it provides additional clinical benefit beyond chemotherapy + PD(L)-1 inhibition or PD(L)-1 blockade alone. Prospective data are limited for this combination in NSCLC patients who progressed on PD(L)-1 blockade.11
Radiation with or without chemotherapy is standard for locally advanced inoperable NSCLC and is sometimes used in the metastatic setting for palliative benefit in conjunction with chemotherapy and immunotherapy. Promising results from a single-arm phase 2 study have stimulated interest in radiation / immune checkpoint combinations in metastatic NSCLC patients12 and led to the design of numerous planned or ongoing clinical trials. Preclinical data suggest synergy between radiation therapy and combined PD(L)-1/ CTLA-4 blockade.13 However, the dose of radiation best suited for this purpose is uncertain. Preclinical studies and clinical observations have suggested that both hypofractionated (high-dose) radiation and low-dose radiation may have favorable immunologic effects such as dendritic cell maturation, homing and activation of T-cells, and macrophage differentiation.13-15
Durvalumab is an IgG1κ monoclonal antibody that inhibits PD-L1 binding to PD-1 and has demonstrated a progression-free and OS benefit when given following definitive chemoradiation in locally advanced NSCLC patients.16 Tremelimumab is an IgG2 antibody that blocks the binding of the inhibitory CTLA-4 receptor to the B7.1 and B7.2 ligands that would otherwise activate T-cells acting through the CD28 receptor. Tremelimumab has been evaluated in combination with durvalumab in multiple settings including first-line metastatic NSCLC (NCT03164616).10,17 As above, preclinical/clinical data suggest potential favorable immune effects both for hypofractionated radiation (8 Gy per fraction) and low-dose radiation (<1 Gy / fraction); however these radiation regimens have not been tested prospectively in human patients in combination with dual PD-L1/CTLA-4 blockade.13-15 Therefore, we performed a multicenter randomised phase 2 study to evaluate the potential benefit of the durvalumab / tremelimumab combination either alone (NoRT) or with low-dose (LDFRT) or hypofractionated radiation (HFRT) in patients with metastatic NSCLC who progressed on prior PD(L)-1 directed therapy.
Methods
Study design and participants
This open-label, multicentre, randomised, phase 2 study (protocol 10021) was conducted by the NCI Experimental Therapeutics Clinical Trials Network (ETCTN) at 18 sites in the United States. A separate cohort of this study enrolling patients with metastatic colorectal cancers was previously reported.15
Patients with histologically confirmed metastatic NSCLC were eligible if they were aged ≥18 years, Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1 with life expectancy greater than 6 months, and had evidence of radiologic or clinical disease progression during previous treatment with systemic PD(L)-1 directed therapy as determined by the treating team; this included patients with innate or acquired resistance to prior PD(L)-1 inhibition. This determination was made clinically, although patients also had to have a biopsy specimen obtained after prior PD(L)-1 therapy and less than 3 months prior to study enrollment for eligibility. Additional inclusion criteria included: measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1), with at least one measurable lesion outside of the intended radiation treatment field in the lung, lymph nodes, adrenal gland or liver; and at least 21 days from prior systemic therapy or radiation. Bone metastases were not permitted to be the target of study radiation. Patients were required to undergo CT of the chest, abdomen, and pelvis and a brain MRI for screening. Patients had to have adequate bone marrow function (absolute neutrophil count of ≥1500 cells per μL, platelet count of ≥100 000 cells per μL, and haemoglobin concentration of ≥9.0 g/dL), adequate liver function (albumin >2.5 g/dL, alanine aminotransferase or aspartate aminotransferase less than 2.5 times the upper limit of normal and total bilirubin level <=1.5 times normal institutional limits), adequate kidney function (measured/calculated creatinine clearance >40mL/min).
Key exclusion criteria included patients who were eligible for approved agents targeting the EGFR, ROS1 or ALK pathway; prior radiation to the intended radiation site or prior radiation that would preclude safely delivering additional radiation as per protocol specifications; prior treatment with CTLA-4 directed therapy; and untreated brain metastases. A complete list of eligibility criteria is in the protocol (appendix).
The protocol was approved by the NCI central institutional review board (cIRB) and cleared by local institutional review boards of all 18 participating centres. The study was carried out in accordance with the protocol, the principles expressed in the Declaration of Helsinki, and applicable regulatory requirements. All patients provided written informed consent in advance of study-specific procedures.
Randomisation and masking
Patients were randomly assigned (1:1:1) to the three arms: durvalumab/tremelimumab alone or in combination with low-dose or hypofractionated radiation. Randomisation followed a permuted block scheme with random block sizes of 3 or 6 and was developed by the study statistician. There were no stratifying factors. Random treatment assignment was conducted using the web-based Theradex Interactive Web Response System (IWRS). Participants and the study team were not masked to group assignment.
Procedures
Imaging and Radiation Oncology Core (IROC) credentialing was required for the most complex radiation modality used at each center as well as for image guided radiation therapy (IGRT). Following randomisation, patients allocated to the radiation treatment arms underwent CT based radiation planning targeting one to two lesions in the lung, lymph nodes, adrenal glands, or liver. Lesions were prioritised according to the following guidelines: 1) lesions progressing on prior PD-1 directed therapy; 2) liver > lung > adrenals > lymph nodes; and 3) the largest feasibly treated lesion that may provide palliative benefit. Radiation techniques are described further in the appendix p1. Our HFRT regimen was based on preclinical evidence that hypofractionated radiation in particular can stimulate secretion of interferon via the cGAS/ STING pathway and increase immunogenicity18 and our LDFRT regimen was supported by prior preclinical studies that demonstrated favorable changes in the tumor microenvironment, specifically regarding macrophage polarization and T-cell infiltration which was maximised at a dose of 0.5 Gy14.
The PD-L1 inhibitor durvalumab was administered intravenously at a fixed dose of 1,500 mg every 4 weeks for a maximum of 13 cycles, and the CTLA-4 inhibitor tremelimumab was administered intraveneously at a fixed dose of 75 mg every 4 weeks for a maximum of four cycles. Dosing of durvalumab/tremelimumab could be temporarily interrupted due to toxicity, but dose reduction was not allowed. Dosing could be resumed if adverse events had been reduced to grade 1 or 0. Treatment was discontinued for disease progression, unacceptable toxicity, or patient or investigator decision to discontinue.
In the LDFRT arm, patients received a dose of 2 Gy administered in four fractions over 2 days (0.5 Gy BID x 2 days) repeated for the first four 28-day cycles of therapy (total dose 8 Gy). In the HFRT arm, patients received a total dose of 24 Gy in three 8 Gy fractions no more frequently than every other day during the first cycle of therapy only. Radiation treatment was started one week following initial durvalumab/tremelimumab administration. Tumor assessments with CT chest, abdomen and pelvis and additionally a brain MRI in patients with a history of brain metastases were performed every 12 weeks after an initial restaging scan at 7–8 weeks. One patient had week-21 scans 31 days out of window, and week-25 treatment 5 days out of window because of travel.
Participants remained on study until death or for at least one year from the time of treatment initiation and at least 12 weeks after removal from study treatment.
Laboratory assessments, including haematology, blood chemistry, and liver and kidney function were done on day 1 (before drug administration) of each treatment cycle. Treatment-related toxicity was assessed throughout the treatment period and every 30 days for 90 days after treatment discontinuation. Toxicities were graded per Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
Tissue for correlative analyses was collected from the screening biopsy or from archival tissue obtained <3 months prior to study enrollment and after progression on prior PD(L)-1 therapy. As specified by a study amendment, PD-L1 immunohistochemistry, multiplex immunofluorescence (mIF), whole exome sequencing, and RNA sequencing were performed through the Cancer Immune Monitoring and Analysis Centers (CIMAC) Immuno-Oncology Biomarkers Network. Detailed procedures are available at https://cimac-network.org/assays/ and as previously described15 and described in more detail in the appendix p1.
Outcomes
The primary outcome of the trial was to compare the overall response rate (ORR, best confirmed response of partial or complete response, locally assessed) using RECIST v1.1 criteria, excluding the irradiated lesion. Secondary endpoints included safety, progression-free survival (PFS) assessed by investigators and overall survival (OS). OS was the interval between study enrollment and death from any cause. For patients lost to follow-up or who had no documentation of death at the time of analysis, follow-up was censored at the last assessment of vital status. PFS was the time from enrollment to the earlier of objective disease progression or death. For patients without progression, follow-up was censored at the date of last adequate restaging, unless death occurred within 12 weeks following the date last known to be progression free, in which case the death was counted as a PFS event. Secondary endpoints of local control and abscopal response were not uniformly collected and are therefore not reported. Prespecified correlative analyses evaluated associations between response and the percentage of tumor cells staining positive for PD-L1 as determined by immunohistochemistry, as well as exploratory analyses evaluating associations between response and infiltrating T-cell populations as by multiplex immunofluorescence as counts of cells per mm2. Additional prespecified secondary correlative and patient reported outcome studies exploring changes in circulating T-cell populations, and spatial/infiltrating immune population/genomic analyses as a result of radiation are ongoing and are therefore not reported.
Statistical analysis
Efficacy and safety analyses were performed in patients who received at least one dose of study therapy. The sample size was based on the primary endpoint of ORR. We estimated a null ORR of approximately 5% for the combination of durvalumab/tremelimumab alone based on preliminary data in a PD-1 refractory population.19 Two pair-wise comparisons of LDFRT and HFRT against the control of NoRT were planned. Since a positive outcome for each comparison in this trial was the superiority of LDFRT or HFRT compared with NoRT, we used one-sided chi-squared tests with 10% type-I errors for each comparison. To assess for early evidence of futility or superiority of NoRT with LDFRT and/or HFRT, each pairwise comparison employed a group sequential design with O’Brien-Fleming stopping boundaries for superiority and Gamma family boundaries (gamma=1) for futility.
A sample size in each comparison of 80 patients (40 per arm) would detect a difference between response rates of 5% and 22% with 81% power (target 80%). Interim analyses were planned after 40 patients within each comparison (20 per arm) had objective response classifications within each comparison. The critical values of the test statistic for superiority of NoRT with either LDFRT or HFRT in each comparison for the interim and final analyses were determined to be 2.054 and 1.317, corresponding to nominal significance levels of 0.02 and 0.094, respectively. In addition, if the p-value at the interim analysis was greater than 0.463, there would be insufficient evidence to reject the null hypothesis and the trial for that comparison would stop early for futility. An interim safety analysis was also performed after 10 patients had been enrolled in each arm, with a plan to stop treatment if any arm had more than 3 patients with dose-limiting toxicities.
Disease control was defined post hoc as having two or more scans with RECIST-defined stable disease (SD), one documented SD followed by non-confirmed partial response (PR), or a confirmed complete or partial response and was assessed by response group. We performed post hoc exploratory analyses of duration of response and of PFS and OS according to prior treatment characteristics (prior RT, intervening therapy, duration of prior PD-1 therapy and interval from prior PD-1 therapy). Response and disease control rates were compared between arms and within clinical subgroups using chi-squared tests (pairwise differences between treatment arms) and Fisher’s exact test (other overall differences). The distributions of PFS, duration of response and OS were estimated using the method of Kaplan-Meier and compared between groups using log-rank tests. Median follow-up was estimated using the Kaplan-Meier method with inverted censor. Hazard ratios were estimated using Cox proportional hazards regression with confidence intervals calculated using log(-log)) and the Efron method for ties. PD-L1 expression and T-cell subsets were associated with response using Wilcoxon rank-sum tests. Post hoc analyses of mutational burden as by whole exome sequencing, and populations of immune cells in the tumor microenvironment as suggested by RNA sequencing were associated with response using Wilcoxon rank-sum tests. Post hoc comparisons of lymphocyte change are based on differences between pre-treatment and 6-week measurements (post-pre). Comparisons according to randomised treatment arm are based on Kruskal-Wallis tests; comparisons according to response are based on Wilcoxon rank-sum tests. , All confidence intervals are 90%, per protocol; statistical significance is defined as p<0.05. Analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
This study was registered with ClinicalTrials.gov, number NCT02888743.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors have had access to all of the data reported in the study.
Results
Between August 24, 2017 and March 29, 2019, 90 patients were enrolled and randomized. 78 subjects received assigned study treatment (26 per arm, Figure 1) and were included in the efficacy and safety analyses. There were 11 deviations due to eligibility: one patient was registered before eligibility screening was completed, 6 patients had negative brain MRI after registration instead of before, one of whom also had a CT abd/pelvis outside the allowable time window. Three patients were registered before brain MRI and then withdrew because of evidence of brain metastases and 1 was registered and then withdrew from the study before MRI evaluation. At the interim analysis, the p-values in both pairwise comparisons exceeded 0.463; therefore, the trial stopped early for futility. Baseline characteristics are shown in Table 1. At the cutoff date for the final analyses (April 12, 2021), median follow up was 12.4 months (IQR: 7.8-15.1) for the entire population.
Figure 1.
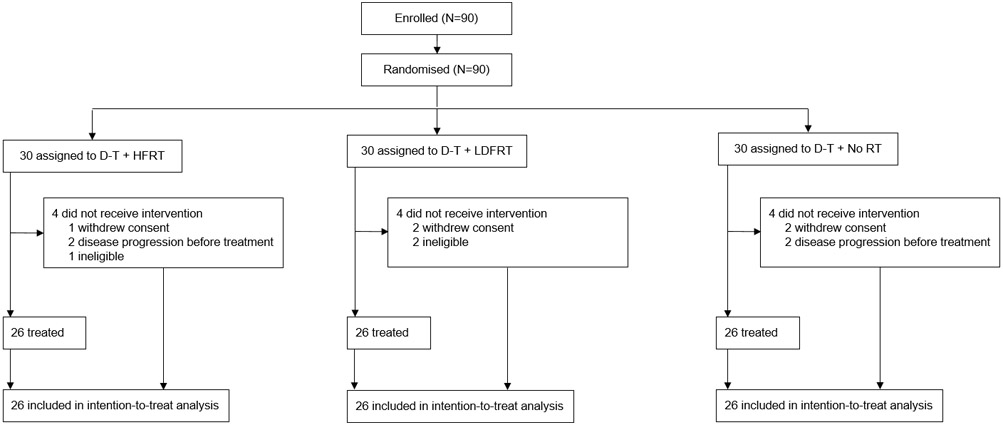
Study CONSORT diagram. D= durvalumab; T- tremelimumab; HFRT- hypofractionacted radiation; LDFRT- low dose fractionated radiotherapy.
Table 1.
Demographics and baseline characteristics of the analytic population
| Durva/treme (n=26) |
Durva/treme Low-dose radiation (n=26) |
Durva/treme Hypofractionated radiation (n=26) |
Total population (n=78) |
|
|---|---|---|---|---|
| Age, years | 65.5 (60-70) | 65.5 (60-73) | 65.1 (57-72) | 66 (59-72) |
| Sex | ||||
| Male | 17 (65) | 18 (69) | 15 (58) | 50 (64) |
| Female | 9 (35) | 8 (31) | 11 (42) | 28 (36) |
| Race | ||||
| African American | 3 (11.5) | 1 (3.8) | 1(3.8) | 5 (6.4) |
| Asian | 1 (3.8) | 3 (11.5) | - | 4 (5.1) |
| White | 21 (80.8) | 22 (84.6) | 22 (84.6) | 65 (83.3) |
| Unknown/not reported | 1 (3.8) | - | 3 (11.5) | 4 (5.1) |
| Ethnicity | ||||
| Hispanic/Latino | - | 1 (3.8) | 3 (11.5) | 4 (5.1) |
| Not Hispanic/Latino | 25 (96.2) | 25 (96.2) | 22 (84.6) | 72 (92.3) |
| Unknown/not reported | 1 (3.8) | - | 1 (3.8) | 2 (2.6) |
| ECOG performance status | ||||
| 0 | 5 (19) | 9 (35) | 6 (23) | 20 (26) |
| 1 | 21 (81) | 17 (65) | 19 (73) | 57 (73) |
| 2 | 1 (4) | 1 (1) | ||
| Histology | ||||
| Adenocarcinoma | 19 (73) | 21 (81) | 16 (62) | 56 (72) |
| Squamous | 3 (12) | 1 (4) | 5 (19) | 9 (12) |
| Not specified | 4(15) | 4 (15) | 5 (20) | 13 (17) |
| PD-L1 percent expression | ||||
| <1% | 4 (15) | 5 (19) | 4 (15) | 13 (17) |
| 1-25% | 7 (27) | 6 (23) | 10 (38) | 23 (29) |
| 25-50% | 1 (4) | 2 (7) | 1 (4) | 4 (5) |
| >50% | 3 (12) | 2 (7) | 3 (12) | 8 (10) |
| Not done | 11 (42) | 11 (42) | 8 (31) | 30 (38) |
| Prior radiation | ||||
| Yes | 20 (77) | 17 (65) | 16 (62) | 53 (68) |
| No | 6 (23) | 9 (35) | 10 (39) | 25 (32) |
| Site prior RT | ||||
| Adrenal | 2 (10) | 2 (12) | 0 (0) | 4 (8) |
| Liver | 2 (10) | 0 (0) | 0 (0) | 2 (4) |
| Lung | 14 (70) | 9 (53) | 6 (38) | 29 (55) |
| Lymph nodes | 2 (10) | 1 (6) | 5 (31) | 8 (15) |
| Not specified | 5 (29) | 5 (31) | 10 (19) | |
| Prior lines of therapy | 3 (2-4) | 3 (2-3) | 3 (2-4) | 3 (2-4) |
| Median Duration of prior PD-1 therapy, weeks | 21.5 (8-44) | 30.1 (16-48) | 27.5 (13-42) | 27.5 (13-45) |
| Time elapsed between prior PD-1 therapy and protocol therapy (months) | 3.4 (1.5-7.4) | 1.6 (1.1-4.8) | 2.4 (1.3-8.4) | 2.5 (1.3-6.9) |
Data are median (IQR), n (%), ECOG = Eastern Cooperative Oncology Group; Durva/treme = durvalumab/tremelimumab
Demographic and clinical characteristics were well balanced between groups at baseline (table 1). The majority of subjects had previously received chemotherapy (66 [85%] of 78) before enrolling on protocol, either as part of initial treatment for localised disease or in the palliative setting. Twenty-five (32%) patients received intervening non-immune therapy (most commonly docetaxel, 6 [8%], or pemetrexed, 6 [8%]). The most common site of radiation in the radiation arms was the lung (32 [62%] of 52), followed by lymph nodes (8 [15%]), liver (6, [12%]) and adrenals (5, [10%]).
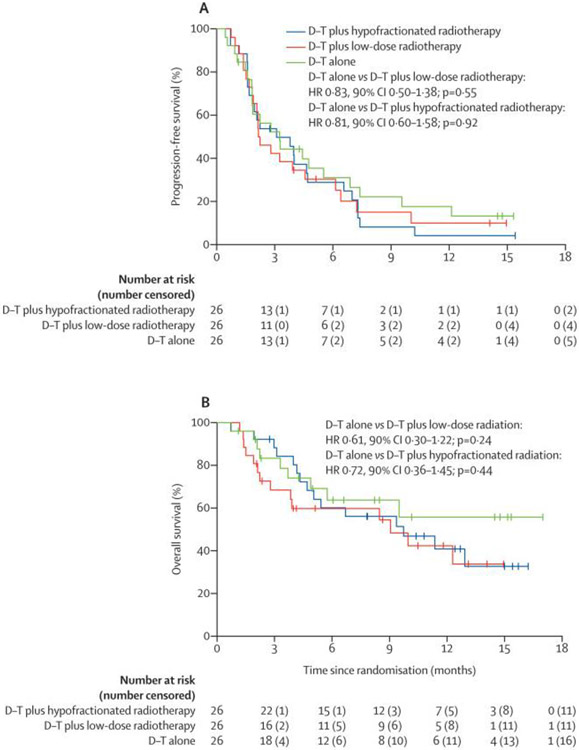
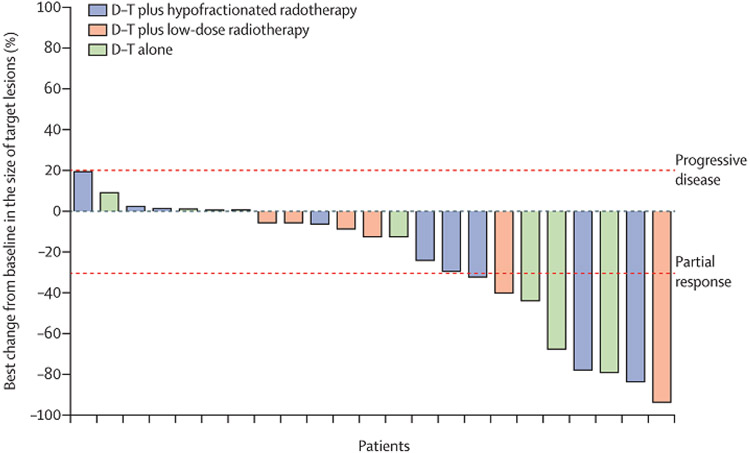
ORR across arms are shown in table 2. PFS events occurred in 67 of 78 patients (figure 2, 85.9%: 24 [92.3%) of 26 HFRT; 22 [84.6%] of 26 LDFRT; 21 [80.8%] NoRT] and there were 39 deaths (50.0%: 15 [57.7%] of 26 HFRT; 15 [57.7%] of 26 LDFRT; 9 [34.6%] of 26 NoRT]. There were no differences in PFS between NoRT and LDFRT or HFRT(figure 2a). OS was also not significantly different between NoRT and LDFRT or HFRT(figure 2b). A post-hoc exploratory evaluation of rates of disease control also revealed no differences between the treatment arms (p=0.74, table 2). Degree of best response among responders or patients with disease control is shown in figure 3.
Table 2:
Best overall response based on investigator assessment by modified RECIST 1.1
| Durva/treme (n=26) |
Durva/treme Low-dose radiation (n=26) |
Durva/treme Hypofractionated radiation (n=26) |
Total population (n=78) |
|
|---|---|---|---|---|
| Best overall response | ||||
| Partial response | 3 (11.5, 1.2-21.8) | 2 (7.7, 0-16.3) | 3 (11.5, 1.2-21.8) | 8 (10.3, 5-18) |
| Stable disease | 11 (42.3) | 12 (46.2) | 10 (38.5) | 33 (42.3) |
| Progressive disease | 10 (38.5) | 8 (30.8) | 10 (38.5) | 28 (35.9) |
| Not Evaluable | 2 (7.7) | 4 (15.4) | 2 (7.7) | 8 (10.3) |
| Pairwise comparison with no radiation arm | ||||
| Difference in response rate | NA | −3.8% (90% CI: −17.3%-9.6%) | 0% (−14.6%, 14.6%) | NA |
| Chi-squared p-value | NA | 0.64 | 0.99 | NA |
| Disease control | 8 (30.8, 15.9-45.7) | 6 (23.1, 9.5-36.7) | 9 (34.6, 19.3-50.0) | 23 (29.5, 21-39) |
| Pairwise comparison with no radiation arm | ] | |||
| Difference in response rate | NA | −7.7% (90% CI: −27.9%-12.5%) | 3.8% (90% CI: −17.5%-25.2%) | NA |
| Chi-squared p-value | NA | 0.53 | 0.77 | NA |
Data are n (%, 90% confidence interval) according to RECIST 1.1=Response Evaluation Criteria in Solid Tumors version 1.1 excluding responses in irradiated lesions. Note: No complete responses were observed.
Figure 2:
Kaplan-Meier estimates of progression free survival (A) and overall survival (B). LDFRT= low-dose fractionated radiation therapy; HFRT = hypofractionated radiation therapy; No RT = no radiation (durvalumab/tremelimumab only).
Figure 3.
Best change in target lesions in patients with disease control or response (n=23). D/T= Durvalumab/tremelimumab; HFRT = hypofractionated radiation; LDFRT = low-dose fractionated radiation therapy
Characteristics of responders are shown in appendix p2. The median duration of response across arms was 10.3 months (90% CI 4.3, infinite, median NoRT not reached, median HFRT not reached, median LDFRT 4.9 months, 90% CI 4.3-5.5 months), with two (66%) of 3 responders in the NoRT arm and one (33%) of 3 responders in the HFRT arm alive and progression-free at the time of last follow up (appendix p3). Post hoc exploratory analyses evaluated prior treatment factors associated with overall and PFS across all arms (appendix p4).
Correlative studies revealed somatic mutations such as TP53 (tumor protein p53) common to NSCLC and a tobacco associated mutational signature (appendix p5-6). Two responding patients had nonsense and missense mutations in the mismatch repair pathway genes MSH3 (mutS homolog 3) (NoRT arm) and POLE (DNA polymerase epsilon, catalytic subunit) (HFRT arm). mRNA sequencing data and multiplex immunofluorescence (mIF) analyses evaluated CD8/PD-1, CD8/PD-1/ki67 and CD4/PD-1/ki67 T-cell populations in relation to response (appendix p7, with significant associations between pretreatment tumor-infiltrating CD8/PD-1+ and CD8/PD-1+/Ki67+ as well as CD4/PD-1+/Ki67+ T-cells (n=52, median (IQR) 37/mm2 (4.1-82.7) versus 4/mm2 (0-17), p=0.03; median 12/mm2 (2.1-44.6) versus 2/mm2 (0-8.0), p=0.04; and median 9/mm2 (2-13.5) versus 1/mm2 (0-3), p=0.01, respectively) as measured by mIF. There were no differences in tumor PD-L1 expression between responders and non-responders (n=49, median (IQR) 2.5% (0-10.0) versus 2% (1-11.2), p=0.69) or when using a 1% cutoff (p=0.32). Post-hoc exploratory analyses of circulating lymphocytes (n=52) identified significant on-treatment changes in the radiation arms (median change (IQR) +0.1% (−2.7 to 2.6) NoRT, −2.6% (−8.0 to - 0.4) LDFRT, and −5.1% (−6.5 to −0.2) HFRT, p=0.007), appendix p8, but no association with radiation target (p=0.85, appendix p9). Decrease in percent lymphocyte count was inversely associated with response across all arms (p=0.003, median change in responders (IQR) +1.7% (−3 to 3.8), median change in non-responders-3.3% (−6.0 to 0.0), appendix, p10).
Overall, 59 of 78 patients (75.6%, 90% exact CI: 66%-83%) experienced toxicity at least possibly related to therapy (19/26 [73.1%] NoRT; 20/26 [76.9%] LDFRT; 20/26 [76.9%] HFRT; Table 3, appendix p11-27). The most common grade 3 or 4 adverse event was dyspnea (2 [8%] of 26 with NoRT; 3 [11%] with LDFRT; and 3 [11%] with HFRT). Toxicities potentially related to treatment occurred in 19 patients treated with NoRT, 20 patients with LDFRT, and 20 patients with HFRT. The most commonly reported potentially related adverse events of any grade were: fatigue (22 of 78, 29%), diarrhea (18, 24%), pruritis (14, 18%), and maculo-papular rash (12, 16%). Fifteen of 78 patients (19%, 90% exact CI: 12%-28%) had grade 3-5 adverse events that were considered at least possibly related to study therapy (4/26 [15%] NoRT; 8/26 [31%] LDFRT; 3/26 [12%} HFRT; p=0.27). Seven patients (9.0%, 90% CI: 4%-16%) discontinued therapy due to drug-related toxicities (1/26 [3.8%] NoRT (colitis); 4/26 [15.4%] LDFRT (increase in lipase and serum amylase, diarrhea, dyspnea, headache and giant cell arteritis syndrome); 2/26 [7.7%] HFRT (adrenal insufficiency (2)). Ten SAEs were reported (1 NoRT; 5 LDFRT; 4 HFRT; appendix p27). There was one grade-5 respiratory failure potentially related to study therapy in a patient treated with LDFRT. This patient received radiation to a subcarinal lymph node and had previously received radiation to the rib and femur. The respiratory failure occurred in the context of disease progression in the lung. Non-treatment related deaths occurred in three (11.5%) of 26 NoRT patients (respiratory failure, multiorgan failure, progressive disease), four (15%) of 26 LDFRT patients (cardiac arrest, death NOS (2), lung cancer) and three (11.5%) of 26 HFRT patients (death NOS, encephalitis, respiratory failure).
Table 3.
All grade 3-5 adverse events regardless of attribution; grade 1-2 adverse events at least possibly related to therapy with at least 10% incidence.
| D/T + HFRT | D/T + LDFRT | D/T + No RT | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-2 | 3 | 5 | 1-2 | 3 | 4 | 5 | 1-2 | 3 | 4 | 5 | |||||||||||||
| % | % | % | % | % | % | % | % | % | % | % | |||||||||||||
| System/Organ Class | Toxicity Description | - | - | 1 | 3.8 | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - |
| Blood and lymphatic system disorders | Anemia | ||||||||||||||||||||||
| Leukocytosis | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Cardiac disorders | Atrial Fibrillation | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - |
| Cardiac Arrest | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | |
| Pericardial Effusion | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Endocrine disorders | Adrenal Insufficiency | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Gastrointestinal disorders | Abdominal Pain | - | - | 1 | 3.8 | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - |
| Ascites | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | |
| Colitis | - | - | 1 | 3.8 | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | |
| Constipation | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | |
| Diarrhea | 4 | 15.4 | 1 | 3.8 | - | - | 6 | 23.1 | 1 | 3.8 | - | - | - | - | 6 | 23.1 | 1 | 3.8 | - | - | - | - | |
| Nausea | 2 | 7.6 | - | - | - | - | 4 | 15.4 | - | - | - | - | - | - | 2 | 7.8 | 1 | 3.8 | - | - | - | - | |
| Vomiting | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | |
| General disorders and administration site conditions | Death NOS | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | 2 | 7.6 | - | - | - | - | - | - | - | - |
| Fatigue | 8 | 30.8 | - | - | - | - | 7 | 26.9 | 1 | 3.8 | - | - | - | - | 6 | 23.1 | 1 | 3.8 | - | - | - | - | |
| Metabolism and Nutrition Disorders | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | |
| Multi-Organ Failure | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | |
| Neck Cellulitis | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Pain | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | |
| Infections and infestations | Encephalitis Infection | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Lung Infection | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | |
| Meningitis | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Urinary Tract Infection | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | |
| Injury, poisoning and procedural complications | Hip Fracture | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - |
| Investigations | Alanine Aminotransferase Increased | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Aspartate Aminotransferase Increased | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | |
| Blood Bilirubin Increased | - | - | 1 | 3.8 | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Gamma-Glutamyl Transferase | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| GGT Increased | - | - | 2 | 7.6 | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Lipase Increased | - | - | 1 | 3.8 | - | - | - | - | - | - | 2 | 7.6 | - | - | - | - | - | - | - | - | - | - | |
| Lymphocyte Count Decreased | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | |
| Serum Amylase Increased | - | - | 1 | 3.8 | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | |
| Metabolism and nutrition disorders | Hypercalcemia | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - |
| Hypoalbuminemia | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Hypokalemia | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | |
| Hyponatremia | - | - | 3 | 11.4 | - | - | - | - | 2 | 7.6 | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | |
| Hypophosphatemia | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Musculoskeletal and connective tissue disorders | Back Pain | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - |
| Generalized Muscle Weakness | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | Metastatic Primary Lung Cancer | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - |
| Progressive Disease | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | |
| Nervous system disorders | Headache | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Recurrent Laryngeal Nerve Palsy | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Spinal Cord Compression | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Psychiatric disorders | Confusion | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - |
| Respiratory, thoracic and mediastinal disorders | Bronchial Hemorrhage | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - |
| Bronchial Obstruction | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | |
| Dyspnea | - | - | 3 | 11.4 | - | - | - | - | 3 | 11.4 | - | - | - | - | - | - | 2 | 7.6 | - | - | - | - | |
| Hypoxia | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Pleural Effusion | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 7.6 | - | - | - | - | |
| Pneumonitis | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | |
| Respiratory Failure | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | - | - | 1 | 3.8 | |
| Sore Throat | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Skin and subcutaneous tissue disorders | Pruritus | 8 | 30.6 | - | - | - | - | 4 | 15.4 | - | - | - | - | - | - | 2 | 7.6 | - | - | - | - | - | - |
| Rash Maculo-Papular | 6 | 23.0 | - | - | - | - | 2 | 7.8 | - | - | - | - | - | - | 3 | 11.5 | 1 | 3.8 | - | - | - | - | |
| Vascular disorders | Hypotension | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Superior Vena Cava Syndrome | - | - | 1 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 3.8 | - | - | - | - | |
| Thromboembolic Event | - | - | 1 | 3.8 | - | - | - | - | 1 | 3.8 | 1 | 3.8 | - | - | - | - | 1 | 3.8 | - | - | - | - | |
Discussion
We conducted a randomised phase 2 study testing the hypothesis that either repeated LDFRT or HFRT would increase the systemic ORR to combined PD-L1 / CTLA-4 blockade in NSCLC patients who previously progressed on PD(L)-1 directed therapy. We didn’t identify any ORR benefit for either radiation regimen (difference in response for LDFRT −3.8% [90% CI: −17.3%-9.6%], Chi-squared p=0.64 and for HFRT difference in response 0% [−14.6%, 14.6%], p=0.99); there were also no differences in PFS and OS between arms. To our knowledge, this represents the only study evaluating the combination of radiation and dual CTLA-4/PD-L1 blockade using a randomised design and testing low-dose radiation (.5 Gy per fraction), and the only randomised study to evaluate the role of radiation as a systemic immune activator exclusively in patients that had progressed on prior PD(L)-1 directed therapy.
Other randomised studies testing the ability of focal radiation to improve systemic response rates to PD-1 inhibition in NSCLC and other histologies have failed to meet their primary endpoints,20-22 although a combined analysis of two trials adding radiation to pembrolizumab demonstrated improved outcomes including progression-free and overall survival.23 While we didn’t demonstrate benefit associated with the addition of radiation to immune checkpoint blockade (ICB), we cannot exclude the possibility that radiation administered in another setting, with different immunotherapy agents, with different timing relative to ICB or using alternative radiation parameters would be beneficial. Although we didn’t observe a difference in response rate according to prior receipt of radiotherapy, we were unable to discriminate prior definitive versus palliative radiation. Additionally, our sample size may have obscured a more limited benefit for the addition of radiotherapy. Our patients received multiple lines of prior therapy and had measurable lesions that remained unirradiated to monitor response; other recent studies treating newly diagnosed or oligometastatic patients and delivering radiation to most or all of the visible tumor volume have demonstrated more promising benefits in pathologic response, PFS and OS.16,24,25
Using the same radiation regimens targeting liver lesions in the colorectal cohort of this trial,15 we also didn’t observe enhancement of response to durvalumab-tremelimumab with radiation. On-treatment biopsies obtained on this cohort demonstrated local infiltration of CD8+ T-cell populations into the tumor microenvironment, accompanied by systemic declines in T-cell populations following liver directed radiotherapy, greater with higher radiation dose. In this NSCLC cohort, despite the difference in disease type and location of the irradiated lesions, we again observed systemic declines in lymphocytes in the radiotherapy arms that were associated with likelihood of progression, which is also consistent with prior retrospective data.26 Although speculatory, it is possible that lymphocyte death resulting from incidental treatment of circulating blood and surrounding normal tissue may have blunted any favorable immunologic effects of radiation. Future studies that limit radiation associated lymphotoxicity or sequence ICB following radiation may be more beneficial and are consistent with recent translational data.27 These factors may explain the failure to improve survival outcomes with either LDFRT or HFRT, despite the promising preclinical data. Preclinical studies may not have fully captured the systemic immune suppressive effects of LDFRT or HFRT administered with clinical techniques; the heterogeneity and immune suppressive nature of advanced metastatic human tumors may have also blunted positive immunological events.
Although not the primary aim of our study, we present data evaluating the safety of combined PD(L)-1/CTLA-4 inhibition in PD(L)-1 inhibitor resistant NSCLC. We find durvalumab / tremelimumab treatment is relatively well-tolerated in this setting; overall rates of grade 3-4 adverse events were favorable compared with a 22% rate that has been observed with durvalumab / tremelimumab in the ARCTIC trial.17 With a median follow up of 12.4 months (impacted by a 9.7 month median survival), we identified patients with prolonged responses and disease control, suggesting durvalumab/tremelimumab can provide meaningful clinical benefit in a subgroup of NSCLC patients who progressed on PD-1 directed therapy. Further studies are warranted to identify the clinical and molecular features associated with benefit in this setting.
These data are consistent with data in metastatic melanoma that suggest that patients who progress on PD(L)-1 directed therapy can respond to combined PD(L)-1 / CTLA-4 blockade.28 The rates of response and disease control in this trial are higher compared with data from another trial testing durvalumab / tremelimumab in a PD(L)-1 resistant population, which demonstrated a 7% response rate.11 In contrast to that study, we allowed intervening systemic therapy following progression on prior PD(L)-1 directed therapy; however, these were the minority of patients included in our study (n=25). It is also notable that we observed a 10% response rate despite the fact that our study population was heavily pretreated and most commonly received initial PD(L)-1 directed therapy following progression on first-line chemotherapy.
Although exploratory and limited to the subset of our cohort with translational samples available, our correlative studies suggest biomarkers of tumor-infiltrating CD8+ and CD4+ T-cells at baseline may predict for response to combined PD-L1/CTLA-4 blockade. This is consistent with the potential ability of combined ICB to reinvigorate a T-cell driven immune response in patients previously treated with PD(L)-1 inhibitors.7 These results are hypothesis generating. Future studies can seek to validate these findings, and could use baseline CD4+ and CD8+ PD-1 and Ki67+ populations to enrich for patients more likely to benefit from combined PD(L)-1 / CTLA-4 therapy, helping to guide patient selection. Decreases in peripheral percent lymphocyte count following treatment were associated with progression; this potential biomarker could be further investigated in future trials. PD-L1 tumor expression levels were not predictive in this setting, consistent with other studies evaluating combined PD(L)-1/CTLA-4 therapy.
Limitations of our study include the variability in the number of lesions and disease burden at baseline, heterogeneity in radiation target/modality/prior PD(L)-1 directed therapy, and inclusion of patients who received intervening therapy following progression on initial PD(L)-1 directed therapy, although our subgroup analyses did not suggest these latter factors had a large impact on our observed outcomes. Exclusively irradiating oligoprogressive lesions or lesions in more suppressive tumor microenvironments such as the liver29 could have had a more positive effect; stratifying by site of irradiation or other factors such as prior radiation administration for prior locoregional disease could have better controlled for these variables. Indeed, we were unable to determine which patients received prior palliative versus definitive locoregional radiation prior to enrolling on trial, a factor that may have impacted outcomes. Serial PET-CT scans were not mandated and are potentially more sensitive for our primary endpoint of RECIST response, especially for detecting response in certain metastatic sites such as bone. We allowed both patients with innate and acquired PD(L)-1 inhibitor resistance and are cognizant that definitions of these groups continue to be refined over time. We did not see a clear impact of duration of prior PD(L)-1 directed therapy on our response and PFS outcomes. Among responding patients, we cannot isolate the effect of PD-L1 versus CTLA-4 inhibition or exclude the possibility that either treatment alone may have been effectual in at least some patients. Radiation planning technique employed by treating physicians may also have differed among patients treated with HFRT versus LDFRT, and unfortunately, local control data were not uniformly captured to compare in-field effects.
In conclusion, our randomised data suggest durvalumab/tremelimumab should be further explored for its ability to benefit NSCLC patients who have progressed on prior PD-L1 therapy. Improved patient selection by T-cell-infiltrated tumors or other markers could be a worthy strategy to try to improve response rates and clinical benefit.
Supplementary Material
Research in context.
There are no randomised data evaluating various radiation doses in combination with PD(L)-1 / CTLA-4 inhibition and in a PD(L)-1 refractory NSCLC population. Furthermore, data evaluating combined PD-L1 / CTLA-4 inhibition with or without radiation in NSCLC patients who have progressed on prior PD(L)-1 therapy are scarce. We conducted a literature search of PubMed for reports published in English up to June 1, 2021 with the terms (“radiation” AND (“PD-1” OR “PD-L1”) AND “CTLA-4” AND “clinical trial”). We identified no prospective published studies fitting these criteria.
Added value of this study
These are among the first published data reporting the efficacy and toxicity of PD(L)-1/CTLA-4 blockade in NSCLC patients resistant to prior PD(L)-1 directed therapy, a growing population of patients in need of novel therapeutic approaches. To our knowledge, this is the first report of a randomised study evaluating the addition of radiation to PD(L)-1/CTLA-4 blockade in any cancer that includes a non-radiation control arm. It is also the first randomised study to evaluate the addition of radiation to immune checkpoint blockade exclusively in PD(L)-1 refractory NSCLC patients, and the first study to prospectively evaluate the effect of low-dose (<1 Gy) radiation in combination with immune checkpoint blockade.
Implications of all the available evidence
PD-L1/CTLA-4 inhibition alone or in combination with radiation led to durable responses in approximately 10% of patients and disease control in 30% of patients and represents a potential treatment option in a subset of PD(L)-1 inhibitor resistant patient population. Our findings and the available evidence do not suggest a benefit for either low-dose or hypofractionated radiation to a single site of disease combined with PD-L1/CTLA-4 inhibition in NSCLC patients resistant to prior PD(L)-1 directed therapy.
Acknowledgments
This study was funded by UM1 CA186709, a Biomarker Supplement to UM1 CA186709, and Center for Immuno-Oncology, Dana-Farber Cancer Institute. Scientific and financial support for the CIMAC-CIDC Network are provided through the National Cancer Institute (NCI) Cooperative Agreements. For this reported study, funding included U24CA224331 (to the Dana-Farber Cancer Institute CIMAC), and U24CA224316 (to the CIDC at Dana-Farber Cancer Institute). The NCI established the Cancer Immune Monitoring and Analysis Centers-Cancer Immunologic Data Commons (CIMAC-CIDC) Network as part of its Cancer Moonshot initiative, to maximize the potential of immunotherapy treatment for cancer. Scientific and financial support for the Partnership for Accelerating Cancer Therapies (PACT) public-private partnership (PPP) are made possible through funding support provided to the FNIH by: AbbVie Inc., Amgen Inc., Boehringer-Ingelheim Pharma GmbH & Co. KG., Bristol-Myers Squibb, Celgene Corporation, Genentech Inc, Gilead, GlaxoSmithKline plc, Janssen Pharmaceutical Companies of Johnson & Johnson, Novartis Institutes for Biomedical Research, Pfizer Inc., and Sanofi.. The study drugs were provided by AstraZeneca.
ES, HS, MA and HXC are employed by NIH.
We thank Manohari Mylsamy and Janice Russell for their invaluable help managing this study team. We acknowledge all participating patients and sites.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing
Individual participant data are not publicly available because this requirement was not anticipated in the study protocol. The study protocol is available in the appendix. Correlative data obtained through the Cancer Immune Monitoring and Analysis Centers (CIMAC) Immuno-Oncology Biomarkers Network will be made available via the Cancer Immunologic Data Commons (CIDC), according to CIMAC-CIDC guidelines.
Declaration of Interests:
JDS: Research support paid to the institution: Merck, BMS, Regeneron, Debiopharm, NCI. Consulting / Scientific Advisory Board / Travel fees / Payment for Lectures: Genentech, Immunitas, Debiopharm, BMS, Nanobiotix, Tilos, AstraZeneca, LEK, Catenion, ACI Clinical, Astellas, Stimit, Merck KGA. Expert witness fees.
Stock options: Immunitas. Equity: Doximity.
AGH: Nothing to declare
SR: Nothing to declare
KZK: Nothing to declare
AL: employee of BMS
JT: Nothing to declare
YL: Nothing to declare
SMA: Research support paid to the institution: Merck, Exilixis, Abbvie, Kura Oncology, Amgen, Nektar.
RCB: Nothing to declare
RDG: Support for present manuscript: NIH/NCI grant. Grants/contracts (to institution): Pfizer, Mirati, Daiichi Sankyo, Jounce Therapeutics, Helsinn, Bristol Myers Squibb, Merck, Janssen, Takeda. Honoraria: Rockpointe CME, Targeted Oncology, OncLive, Society for Immunotherapy of Cancer. Travel for meetings: Pfizer, AstraZeneca. Advisory boards: Mirati, Daiichi Sankyo, AstraZeneca, Sanofi, Oncocyte, Jazz Pharmaceuticals, BluePrint Medicines, Pfizer. Co-chair, Hoosier Cancer Research Network Thoracic Clinical Trial Working Group
CL: Honorarium for chairing the DSMB for Delcath, Inc.
JH: Advisory boards: Bayer, Merck. Research funding: Merck, Boston Biomedical, Treos Bio, Senhwa Biosciences, Bayer, Incyte, TriOncology, Seattle Genetics, Hutchison MediPharma, Pionyr Immunotherapeutics, Trovogene, Taiho Pharmaceutical, Effector Therapeutics, G1 Therapeutics.
JLA: Research support from Pfizer, Scientific Advisory Board membership for the Lustgarten Foundation, Stand Up to Cancer, Moleculin Biotech, Bessor Pharma, and Fujifilm; Stock Options from Bessor Pharma, Moleculin Biotech; DSMB membership honoraria from Panbela Therapeutics and the Pancreatic Cancer Action Network.
SKJ: Grant funding to institution from NCI/CTEP. Consultant / adjudication committee for Merck, IMX Medical, and Syntactx.
NVU: Consulting: QED, Ipsen, Taiho, Incyte, AstraZeneca, Astellas. Research Funding: Taiho Inc, Eli Lilly, Ipsen, EMD Serono. Long position holdings: Natera, Exact Sciences
KLS: DSMC membership- Case Comprehensive Cancer Center
JMJ: Consulting for Foundation Medicine
HP: Research grants/funding to institution from: Adlai Nortye USA, Alpine Immune Sciences, Ambrx, Amgen, Aprea Therapeutics AB, ArrayBioPharma, Bayer, BeiGene, BJ Bioscience, Bristol-Myers Squibb, Daiichi Pharmaceutical, Eli Lilly, Elicio Therapeutics, EMD Serono, Exelixis, Genentech, Gilead Sciences, GlaxoSmithKline, Gossamer Bio, Hoffman-LaRoche, Hutchison MediPharma, ImmuneOncia Therapeutics, Incyte, Jounce Therapeutics , Mabspace Biosciences, MacroGenics, Medimmune, Medivation, Merck, Millennium, Mirati Therapeutics, Novartis Pharmaceuticals, Oncologie, Pfizer, PsiOxus Therapeutics, Puma Biotechnology, Regeneron Pharmaceuticals, RePare Therapeutics, Seattle Genetics, Synermore Biologics, Taiho Pharmaceutical, TopAlliance Biosciences, TurningPoint Therapeutics, Vedanta Biosciences, XencorIn. Meetings/Travel: Daiichi Sankyo, Vedanta
LCV: Consulting fees for Janssen, BMS, Takeda, Jazz, and Daiichi Sankyo.
ES: Nothing to declare
HS: Nothing to declare
MMA: Nothing to declare
HL: Nothing to declare
CC: Nothing to declare
NL: Nothing to declare
AJ: Nothing to declare
LY: Nothing to declare
JA: Nothing to declare
LG: Nothing to declare
JLW: Research support from NIH/NCI.
RHM: Research support: ViewRay, AstraZeneca. Scientific Advisory Board: ViewRay, AstraZeneca. Expert witness fees.
MMA: Consultant for: Genentech, Bristol-Myers Squibb, Merck, AstraZeneca, Maverick, Blueprint Medicine, Syndax, Ariad/Takeda, Nektar, Gritstone, ArcherDX, Mirati, NextCure, Novartis, EMD Serono, Panvaxal/NovaRx. Research funding (to institute): AstraZeneca, Lilly, Genentech, Bristol-Myers Squibb, Merck. Participation on Data Safety Monitoring Board/Advisory Board for Bristol-Meyers Squibb, Apollomics.
SR: Research support from Bristol-Myers-Squibb, Merck, Affimed, and KITE/Gilead. SR is on the SAB of Immunitas Therapeutics and has equity in the company.
HXC: no disclosures
AMM: Research support: Merck, BMS, Transgene, Incyte, Trisalus, Genentech, NIH. Consulting fees: Zosano. Advisory board: BMS, Astra-Zeneca, Incyte, Dynavax. Stock options: MultiplexThera.
CJW: Equity in BioNTech, receives U24 research support from NIH/NCI, and receives research funding from Pharmacyclics, Inc.
FSH: Research support from NCI/NIH, Bristol-Meyers Squibb, Novartis, Genentech. Royalties/licenses: Bristol-Meyers Squibb, Novartis. Consulting fees: Bristol-Meyers Squibb, EMD Serono, Surface, Sanofi, Genentech, Gossamer, Trillium, Immunocore, Merck, Novartis, Compass Therapeutics, Pieris, Bioentre, Iovance, Catalym, Amgen. Patents: Methods for Treating MICA-Related Disorders (#20100111973) with royalties paid, a patent Tumor antigens and uses thereof (#7250291) issued, a patent Angiopoiten-2 Biomarkers Predictive of Anti-immune checkpoint response (#20170248603) pending, a patent Compositions and Methods for Identification, Assessment, Prevention, and Treatment of Melanoma using PD-L1 Isoforms (#20160340407) pending, a patent Therapeutic peptides (#20160046716) pending, a patent Therapeutic Peptides (#20140004112) pending, a patent Therapeutic Peptides (#20170022275) pending, a patent Therapeutic Peptides (#20170008962) pending, a patent Therapeutic Peptides (Patent number: 9402905) issued, a patent METHODS OF USING PEMBROLIZUMAB AND TREBANANIB pending, a patent Vaccine compositions and methods for restoring NKG2D pathway function against cancers (Patent number: 10279021) issued, a patent Antibodies that bind to MHC class I polypeptide-related sequence A (Patent number: 10106611) issued, and a patent ANTI-GALECTIN ANTIBODY BIOMARKERS PREDICTIVE OF ANTI-IMMUNE CHECKPOINT AND ANTI-ANGIOGENESIS RESPONSES (Publication number: 20170343552) pending. Data Safety Monitoring Board/Advisory Board: Aduro, Checkpoint Therapeutics. Leadership: Bicara, Apricity. Stock options: Checkpoint Therapeutics, Pionyr, Apricity, Bicara.
Contributor Information
Jonathan D Schoenfeld, Department of Radiation Oncology, Brigham and Women’s Hospital, Boston, MA, USA; Department of Radiation Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Anita Giobbie-Hurder, Department of Data Science, Dana-Farber Cancer Institute, Boston, MA, USA.
Srinika Ranasinghe, Center for Immuno-Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Katrina Z Kao, Center for Immuno-Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Ana Lako, Center for Immuno-Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Junko Tsuji, Genomics Platform, Broad Institute, Cambridge, MA, USA.
Yang Liu, Department of Data Science, Dana-Farber Cancer Institute, Boston, MA, USA.
Ryan C Brennick, Center for Immuno-Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Ryan D Gentzler, Division of Hematology/Oncology, University of Virginia Cancer Center, Charlottesville, VA, USA.
Carrie Lee, Division of Hematology/Oncology, University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, NC, USA.
Joleen Hubbard, Department of Medical Oncology, Mayo Clinic, Rochester, MN, USA.
Susanne M Arnold, Division of Medical Oncology, University of Kentucky Markey Cancer Center, Lexington, KY, USA.
James L Abbruzzese, Division of Medical Oncology, Duke Cancer Institute, Durham, NC, USA.
Salma K Jabbour, Department of Radiation Oncology, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, USA.
Nataliya V Uboha, Department of Medicine, University of Wisconsin Carbone Cancer Center, University of Wisconsin School of Medicine, Madison, WI, USA.
Kevin L Stephans, Department of Radiation Oncology, Cleveland Clinic, Cleveland, OH, USA.
Jennifer M Johnson, Department of Medical Oncology, Sidney Kimmel Cancer Center – Jefferson Health, Philadelphia, PA, USA.
Haeseong Park, Division of Oncology, Siteman Cancer Center, Washington University, Saint Louis, MO, USA.
Liza C Villaruz, Division of Hematology/Oncology, University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh, PA, USA.
Elad Sharon, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD, USA.
Howard Streicher, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD, USA.
Mansoor M Ahmed, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD, USA.
Hayley Lyon, Genomics Platform, Broad Institute, Cambridge, MA, USA.
Carrie Cibuskis, Genomics Platform, Broad Institute, Cambridge, MA, USA.
Niall Lennon, Genomics Platform, Broad Institute, Cambridge, MA, USA.
Aashna Jhaveri, Department of Data Science, Dana-Farber Cancer Institute, Boston, MA, USA.
Lin Yang, Department of Data Science, Dana-Farber Cancer Institute, Boston, MA, USA.
Jennifer Altreuter, Department of Data Science, Dana-Farber Cancer Institute, Boston, MA, USA.
Lauren Gunasti, Department of Radiation Oncology, Brigham and Women’s Hospital, Boston, MA, USA; Department of Radiation Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Jason L Weirather, Department of Data Science, Dana-Farber Cancer Institute, Boston, MA, USA.
Raymond H Mak, Department of Radiation Oncology, Brigham and Women’s Hospital, Boston, MA, USA; Department of Radiation Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Mark M Awad, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Scott J Rodig, Center for Immuno-Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Helen X Chen, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD, USA.
Catherine J Wu, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Arta M Monjazeb, Department of Radiation Oncology, University of California Davis Comprehensive Cancer Center, Sacramento, CA, USA.
F Stephen Hodi, Center for Immuno-Oncology, Dana-Farber Cancer Institute, Boston, MA, USA; Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
References
- 1.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. The New England journal of medicine 2018. [DOI] [PubMed] [Google Scholar]
- 2.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. The New England journal of medicine 2018. [DOI] [PubMed] [Google Scholar]
- 3.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine 2016; 375(19): 1823–33. [DOI] [PubMed] [Google Scholar]
- 4.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. The New England journal of medicine 2018; 378(24): 2288–301. [DOI] [PubMed] [Google Scholar]
- 5.Schoenfeld AJ, Hellmann MD. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020; 37(4): 443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gettinger SN, Wurtz A, Goldberg SB, et al. Clinical Features and Management of Acquired Resistance to PD-1 Axis Inhibitors in 26 Patients With Advanced Non-Small Cell Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2018; 13(6): 831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei SC, Levine JH, Cogdill AP, et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell 2017; 170(6): 1120–33 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. The New England journal of medicine 2019. [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. The Lancet Oncology 2021; 22(2): 198–211. [DOI] [PubMed] [Google Scholar]
- 10.Rizvi NA, Cho BC, Reinmuth N, et al. Durvalumab With or Without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA oncology 2020; 6(5): 661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leighl NB, Redman MW, Rizvi N, et al. Phase II study of durvalumab plus tremelimumab as therapy for patients with previously treated anti-PD-1/PD-L1 resistant stage IV squamous cell lung cancer (Lung-MAP substudy S1400F, NCT03373760). J Immunother Cancer 2021; 9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nature medicine 2018; 24(12): 1845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015; 520(7547): 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klug F, Prakash H, Huber PE, et al. Low-Dose Irradiation Programs Macrophage Differentiation to an iNOS(+)/M1 Phenotype that Orchestrates Effective T Cell Immunotherapy. Cancer Cell; 2013. p. 589–602. [DOI] [PubMed] [Google Scholar]
- 15.Monjazeb AM, Giobbie-Hurder A, Lako A, et al. A Randomized Trial of Combined PD-L1 and CTLA-4 Inhibition with Targeted Low-Dose or Hypofractionated Radiation for Patients with Metastatic Colorectal Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2021; 27(9): 2470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. The New England journal of medicine 2018. [DOI] [PubMed] [Google Scholar]
- 17.Planchard D, Reinmuth N, Orlov S, et al. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2020; 31(5): 609–18. [DOI] [PubMed] [Google Scholar]
- 18.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nature communications 2017; 8: 15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garon EB, Spira AI, Goldberg SB, et al. Safety and activity of durvalumab + tremelimumab in immunotherapy (IMT)-pretreated advanced NSCLC patients. Journal of Clinical Oncology 2018; 36(15_suppl): 9041-. [Google Scholar]
- 20.McBride S, Sherman E, Tsai CJ, et al. Randomized Phase II Trial of Nivolumab With Stereotactic Body Radiotherapy Versus Nivolumab Alone in Metastatic Head and Neck Squamous Cell Carcinoma. J Clin Oncol 2020: JCO2000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theelen W, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA oncology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh J, Menon H, Chen D, et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer 2020; 8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theelen W, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med 2021; 9(5): 467–75. [DOI] [PubMed] [Google Scholar]
- 24.Altorki NK, McGraw TE, Borczuk AC, et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. The Lancet Oncology 2021. [DOI] [PubMed] [Google Scholar]
- 25.Bauml JM, Mick R, Ciunci C, et al. Pembrolizumab After Completion of Locally Ablative Therapy for Oligometastatic Non-Small Cell Lung Cancer: A Phase 2 Trial. JAMA oncology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pike LRG, Bang A, Mahal BA, et al. The Impact of Radiation Therapy on Lymphocyte Count and Survival in Metastatic Cancer Patients Receiving PD-1 Immune Checkpoint Inhibitors. International journal of radiation oncology, biology, physics 2019; 103(1): 142–51. [DOI] [PubMed] [Google Scholar]
- 27.Wei J, Montalvo-Ortiz W, Yu L, et al. Sequence of alphaPD-1 relative to local tumor irradiation determines the induction of abscopal antitumor immune responses. Sci Immunol 2021; 6(58). [DOI] [PubMed] [Google Scholar]
- 28.Pires da Silva I, Ahmed T, Reijers ILM, et al. Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: a multicentre, retrospective, cohort study. The Lancet Oncology 2021. [DOI] [PubMed] [Google Scholar]
- 29.Yu J, Green MD, Li S, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nature medicine 2021; 27(1): 152–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.