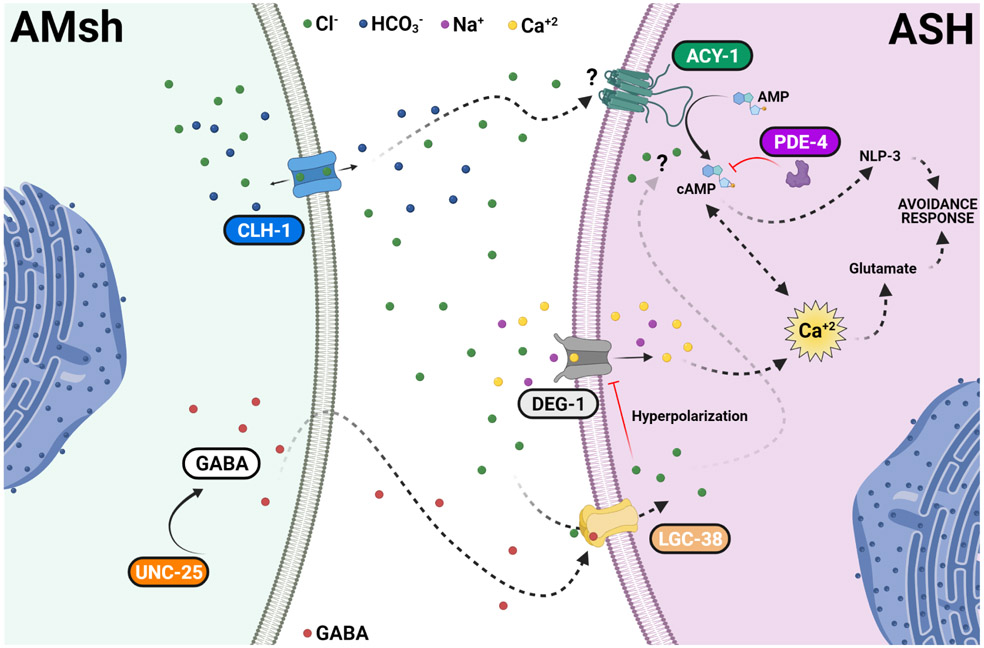
Fig. 8. Regulation of touch neurons’ function by glial Cl− channel CLH-1 in C. elegans.
Activation of DEG/ENaC channel DEG-1 by mechanical forces causes depolarization of ASH which is expected to activate voltage-gated Ca2+ channel EGL-19, leading to elevation of intracellular Ca2+ concentrations ([Ca2+]i). Elevation of [Ca2+]i causes the release of glutamate. CLH-1 is expressed in AMsh glia and mediates Cl− flux needed for GABA signaling. GABA activation of the GABAA receptor LGC-38 causes hyperpolarization of the ASH touch neuron’s plasma membrane. This hyperpolarization closes EGL-19 leading to a decrease in the [Ca2+]i, and thus, in glutamate release. A second pathway involving cAMP synthesis by adenylyl cyclase ACY-1 and degradation by phosphodiesterase PDE-4 is also influenced by AMsh glia CLH-1. This regulation might be either directly mediated by Cl− on the activity of ACY-1 or indirectly mediated by Ca2+ inhibition of ACY-1. cAMP leads to PKA activation which in turn enhances the release of dense core vesicles containing the neuropeptide NLP-3. PKA may also contribute to Ca2+ buffering by activation of the Na+/Ca2+ exchanger or to the hyperpolarization of the plasma membrane by activation of K+ channels. The lack of CLH-1 in AMsh glia leads to higher levels of [Ca2+]i (caused by lack of hyperpolarization), hence, to a higher release of glutamate and inhibition of ACY-1, which in turn decreases NLP-3 release. Knockout of clh-1 may also directly decrease ACY-1 activity. Blue arrows indicate activation. Red lines indicate inhibition. Black arrows indicate transport of ions across the channels. Dashed labels and lines indicate suggested proteins and pathways. Ions and GABA legends are indicated in the figure.