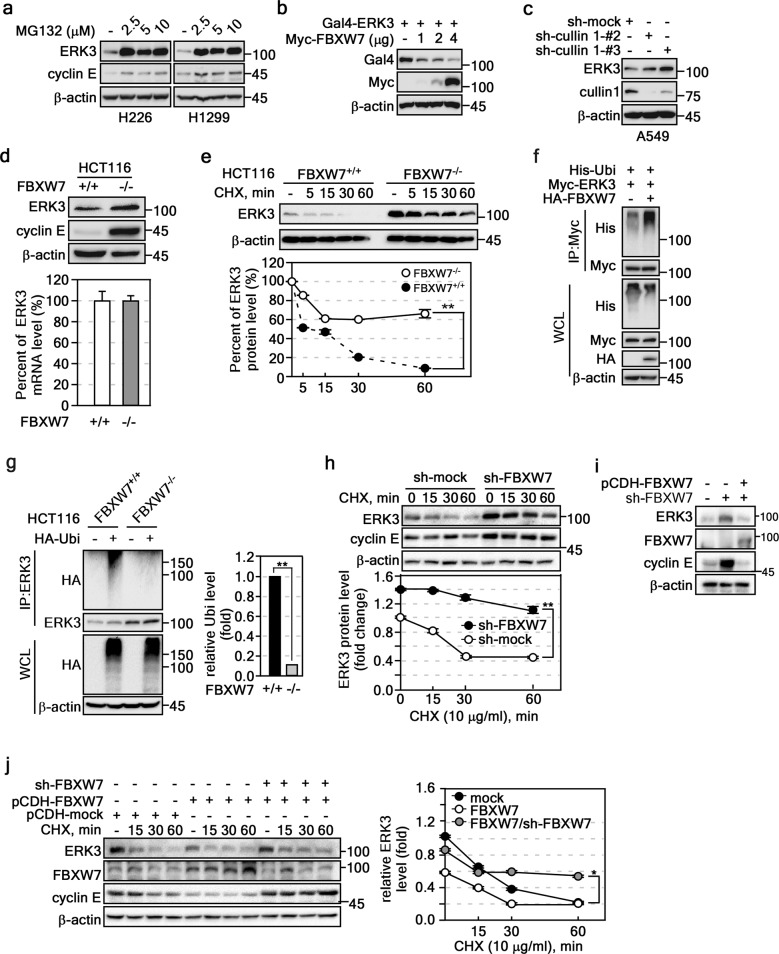
Fig. 2. FBXW7 induced the ubiquitination of ERK3.
a MG132 restored ERK3 protein levels. Lung cancer cells (H226 and H1299 cells) were treated with MG132 (10 μM) for 4 h, and cell lysates (30 μg) were subjected to Western blotting to assess ERK3 protein levels. b FBXW7 downregulated ERK3 protein levels. FBXW7-mediated ERK3 protein level changes were assessed by Western blotting (30 μg protein/lane). c Cullin 1 knockdown increased ERK3 protein levels. ERK3 protein levels in cullin-knockdown cells (30 μg protein/lane) were assessed by Western blotting. d Upper panels, FBXW7 deficiency increased ERK3 protein levels. ERK3 protein levels in cell lysates (30 μg) of HCT116FBXW7+/+ and HCT116FBXW7−/− cells were determined by Western blotting. Cyclin E: positive control. Graphs, ERK3 gene expression was not involved in ERK3 destabilization. ERK3 mRNA levels in HCT116FBXW7+/+ or HCT116FBXW7−/− cells were evaluated by real-time PCR. e FBXW7 attenuated ERK3 degradation. Upper panels, ERK3 protein levels in HCT116FBXW7+/+ and HCT116FBXW7−/− cells were determined by Western blotting (30 μg cell lysate/lane, CHX; 10 μg/ml). Graphs, The ERK3 band intensities were normalized to the β-actin intensity. f FBXW7 increased ERK3 ubiquitination. FBXW7-mediated ERK3 ubiquitination was evaluated by IP (400 μg cell lysate)/Western blotting. g Depletion of FBXW7 suppressed ERK3 ubiquitination. Left panels, The endogenous ERK3 ubiquitination content in HCT116FBXW7+/+ and HCT116FBXW7−/− cells was evaluated by IP (500 μg cell lysate/lane)/Western blotting. Graphs, The intensities of HA-Ubi ERK3 bands were measured and normalized using ImageJ (ver. 1.52a). h Upper panels, FBXW7 knockdown attenuated ERK3 degradation. The ERK3 turnover rate in FBXW7-knockdown A549 cells was evaluated by Western blotting (20 μg cell lysate/lane, CHX; 10 μg/ml). The ERK3 band intensities were normalized to the β-actin intensity. Cyclin E, positive control. i FBXW7 reintroduction into FBXW7-knockdown cells suppressed ERK3 protein levels. The ERK3 protein levels in A549 stable cells stably expressing sh-mock, sh-FBXW7, or sh-FBXW7/Lenti-pCDH-FBXW7 were determined by Western blotting (20 μg cell lysate/lane). j Left panels, FBXW7 knockdown decreased the ERK3 turnover rate, which was increased by FBXW7 overexpression. The ERK3 turnover rate in A549 cells stably expressing Lenti-pCDH-FBXW7 or Lenti-pCDH-FBXW7/sh-FBXW7 was determined by Western blotting (20 μg cell lysate/lane, CHX; 10 μg/ml). Graphs, The ERK3 band intensities were normalized to the β-actin intensity. a-j β-Actin was used as the internal control to ensure equal protein loading. a-j The data are from three independent experiments. d, e, g, h, j The values are shown ± the SEMs. Significance; *p < 0.05, **p < 0.01 vs. indicated control by Student’s t test. f, g WCL, whole-cell lysate.