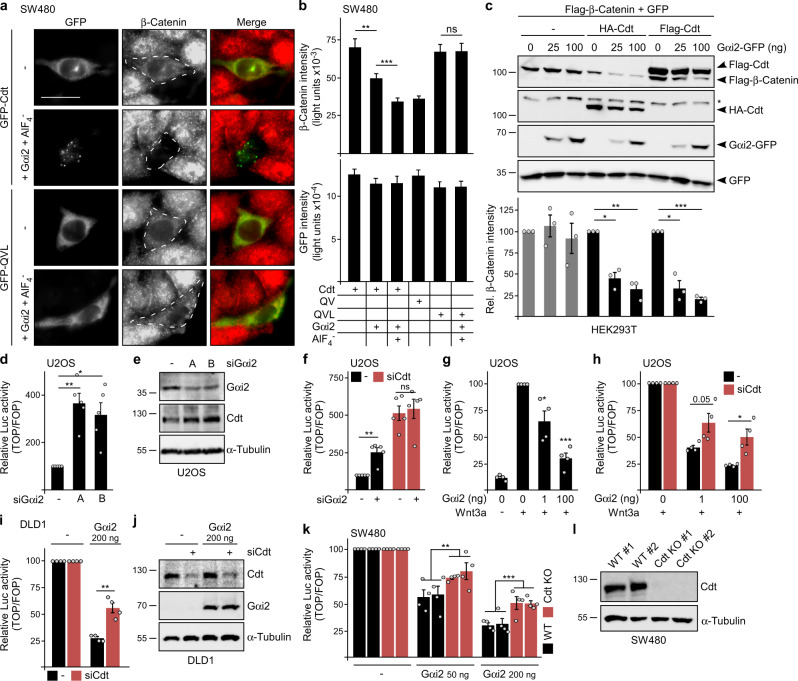
Fig. 4. Gαi2 promotes conductin-mediated inhibition of Wnt signaling.
a Immunofluorescence staining of endogenous β-catenin (red) in SW480 cells, which were transfected and treated as indicated on the left. Scale bar: 20 µm. b Quantification of β-catenin and GFP fluorescence intensity in four independent experiments as in (a). c Western blotting for Flag, HA, and GFP in lysates of HEK293T cells transfected as indicated above the blots. GFP: loading and transfection control. * indicates unspecific band. Quantification of Flag-β-catenin normalized to GFP of three independent experiments. d, f–h Luciferase activity (TOP/FOP) in U2OS cells transfected with indicated siRNAs (d, f), or/and indicated amounts of Gαi2 (g, h). Wnt3a treatment is indicated. e Western blotting showing the efficiency of Gαi2 knockdown in U2OS cells. Consistent with TOP-Flash activation (d), expression of the β-catenin target gene conductin (Cdt) was increased upon knockdown. α-Tubulin: loading control. i, k Luciferase activity (TOP/FOP) in Gαi2 transfected DLD1 cells without (–) and with conductin knockdown (i), and in parental SW480 cells, a WT control clone and two CRISPR/Cas9 AXIN2/Conductin knockout clones (Cdt KO) (k). To facilitate comparisons of the Gαi2 effects in cells with (black bars) and without conductin (siCdt/Cdt KO, red bars) in (h, i, k), the initial luciferase activities without Gαi2 were set to 100% for both conditions, and the luciferase activities with Gαi2 are presented relative to the respective initial activity. j, l Western blotting showing conductin knockdown and Gαi2 expression in the DLD1 cells used in (i, j), and loss of conductin expression in the SW480 knockout clones used in (k, l). α-Tubulin: loading control. Results are mean ± SEM (n = 80 [b], n = 3 [c], n = 5 [d, f], n = 4 [g, h, i, k]). *p < 0.05, **p < 0.01, ***p < 0.001 (two-sided Student’s t-test). Molecular weight is indicated in kDa (c, e, j, l). Source data and exact p-values are provided as source data file.