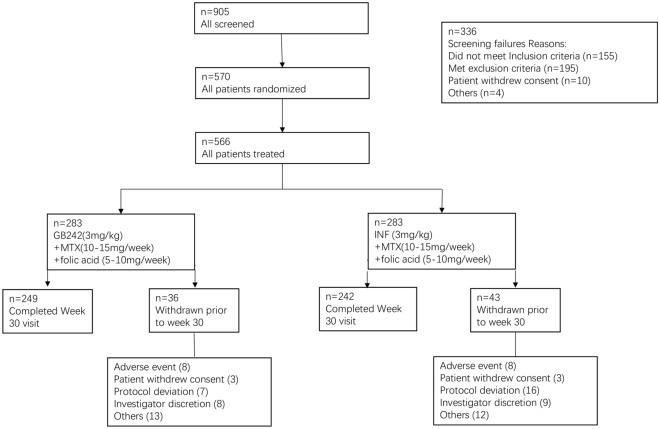
Fig. 1.
Disposition flow chart of the study population. A total of 905 patients were screened for the study, and 566 eligible patients were randomized into a GB242 group (n = 283) or an infliximab reference product (INF) group (n = 283) to receive 3 mg/kg of GB242 or INF, respectively, coadministered with methotrexate (MTX) and folic acid. The full analysis set (FAS) for GB242 is n = 283 and infliximab reference product (INF) n = 283. The per-protocol set (PPS) for GB242 is n = 237 and INF n = 233