Abstract
Pulmonary arteriovenous malformation (AVM) is a congenital vascular disease in which interventional radiologists can play both diagnostic and therapeutic roles in patient management. The diagnosis of pulmonary AVM is simple and can usually be made based on CT images. Endovascular treatment, that is, selective embolization of the pulmonary artery feeding the nidus of the pulmonary AVM, and/or selectively either the nidus or draining vein, has become a first-line treatment with advances in interventional devices. However, some vascular diseases can simulate pulmonary AVMs on CT and pulmonary angiography. This subset can confuse interventional radiologists and referring physicians. Vascular mimickers of pulmonary AVM have not been widely known and described in detail in the literature, although some of these require surgical correction, while others require regular follow-up. This article reviews the clinical and radiologic features of pulmonary AVMs and their mimickers.
Keywords: Arteriovenous malformations; Aneurysm; Lung; Pleura; Thoracic wall; Diagnosis, differential; Scimitar syndrome; Stenosis, pulmonary vein
INTRODUCTION
Pulmonary arteriovenous malformation (AVM) is a vascular conglomerate that consists of feeding arteries, nidus, and draining veins. A nidus indicates a direct communication between the pulmonary artery and veins without a capillary network. Accurate diagnosis and proper management are essential, as AVM can be associated with significant morbidity and mortality, such as brain abscesses, cerebral stroke, and massive pulmonary hemorrhage. Currently, pulmonary AVMs are incidentally detected during a medical checkup, and an imaging diagnosis is usually made based on contrast-enhanced chest CT. However, a group of rare vascular lesions is often misdiagnosed as pulmonary AVM on CT images, which may eventually lead to conventional pulmonary angiography for embolization (Fig. 1, Table 1) [1,2,3,4]. In the literature, approximately 10% of pulmonary AVMs diagnosed on CT images were proven to have no fistula between the pulmonary artery and vein on conventional angiography. Of these, two-fifths are pulmonary vascular anomalies other than pulmonary AVM, and the remaining three-fifths are pulmonary parenchymal nodules [5]. This review aimed to introduce these vascular lesions and describe their characteristic CT and angiographic findings.
Fig. 1. Illustration of the vascular mimickers of pulmonary AVM.
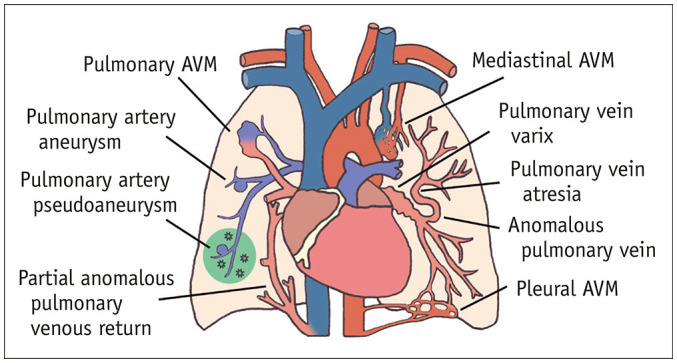
AVM = arteriovenous malformation
Table 1. Summary of Various Vascular Lesions Which Can Be Confused with Pulmonary Arteriovenous Malformation.
| Clinical Features | Radiologic Features | Management | |
|---|---|---|---|
| Pulmonary arteriovenous malformation | Dyspnea, hemoptysis, cerebral stroke, brain abscess | Well-defined peripheral lung nodule/mass, clustered dilated pulmonary vessels | Endovascular embolization of feeding artery |
| Extrapulmonary arteriovenous malformation | Pulsating bulging mass of chest wall | Vascular tangle abutting pleural surface, systemic feeding arteries and draining veins | Endovascular sclerotherapy of nidus |
| Anomalous pulmonary vein | Dyspnea, respiratory distress, hemoptysis | Anomalous vein which drains into the left atrium involving unilateral lung, lobe, segment or subsegment | Observation |
| Pulmonary vein atresia or stenosis | Tachypnea, dyspnea, recurrent pneumonia, hemoptysis | Venous drainage from the affected lung through various forms of collaterals to the left atrium | Surgical repair or balloon angioplasty |
| Pulmonary vein varix | Cough, dyspnea, palpitation, hemoptysis, cerebral stroke (rare) | Dilated anomalous perihilar pulmonary vein without arterial dilatation or arteriovenous fistula | Depends on the underlying cause; surgical resection |
| Partial anomalous pulmonary venous drainage | Dyspnea, heart murmur, arrhythmia, palpitation | Anomalous vein which drains into the systemic vein involving at least one pulmonary vein, but not all | Surgical repair; endovascular treatment in selected case |
| Pulmonary artery aneurysm or ectasia | Dyspnea, chest pain, palpitation, syncopal episodes, hoarseness | Focal or diffuse dilatation of main pulmonary artery and/or branches | Surgical repair; endovascular treatment in selected case |
Anatomy
Relevant Embryology
A thorough understanding of embryology is important to understand the possible causes of pulmonary vascular anomalies. In the early stage (the first two months) of fetal development, the blood flow from the primitive lung buds drains into the splanchnic plexus, which is connected to the systemic venous system (cardinal and the umbilicovitelline veins) (Supplementary Fig. 1) [6]. An outpouching from the dorsal wall of the left atrium forms the common pulmonary vein and communicates with the splanchnic plexus, thus forming the initial route of pulmonary venous drainage. The cardinal and umbilicovitelline veins involute, thus separating the two venous systems. The common pulmonary vein is finally incorporated into the left atrium, forming four pulmonary veins. Any alterations in this process can cause various types of pulmonary venous anomalies.
The embryogenic phase of pulmonary arterial development begins with the formation of an outflow tract from the bulbus cordis and truncus arteriosus. During the fifth and sixth weeks of embryonic life, a helical-shaped aortopulmonary septum is created to divide the outflow tract into the aorta and pulmonary artery [7]. The ventral segment of the sixth aortic arch forms the bilateral proximal pulmonary arteries, whereas the left dorsal arch forms the ductus arteriosus (Supplementary Fig. 2). The distal pulmonary arteries are derived from primitive buds that come from these arches and grow toward the inside of the lung buds to later anastomose with them. The ductus arteriosus connects the proximal left pulmonary artery to the undersurface of the isthmus of the aorta, which closes and fibroses to form the ligamentum arteriosum.
Pulmonary Veins and Arteries
The pulmonary veins are large blood vessels that carry oxygenated blood from both lungs and drain into the left atrium [6,8]. The usual arrangement consists of four separate pulmonary veins that drain individually into the left atrium. The right superior pulmonary vein drains the right upper and middle lobes. The left superior pulmonary vein drains the lingual and left upper lobes. The inferior pulmonary veins drain into the lower lobes. This anatomical arrangement is present in 60%–70% of the population.
The main pulmonary artery, which stems from the right ventricle, may have a diameter as large as 28 mm [9]. The right pulmonary artery passes anteriorly to the main bronchus and divides into the truncus anterior and interlobar branches, which is the most common pattern. The distal left pulmonary artery arch over and behind the left main bronchus. Anatomic variations in the branching pattern on the left side were far more common than on the right. The bilateral individual pulmonary arteries should have a similar caliber.
AVMs
Pulmonary AVM
Pulmonary AVMs affect 1 in 2500 (95% confidence interval: 1 in 1300 to 1 in 5600), and over 80% of these are associated with hereditary hemorrhagic telangiectasia [3]. The etiology of pulmonary AVM remains unclear. However, germline mutations in two major genes (ENG and ACVRL1) in the transforming growth factor-beta pathway, which were recently discovered by genetic analyses of hereditary hemorrhagic telangiectasia, may be related to the development of abnormal communication between the pulmonary arteries and veins [10,11]. Pulmonary AVMs can be divided into simple and complex types according to the number of feeding pulmonary arteries. A simple pulmonary AVM is the most frequent (accounting for approximately 80%), consisting of a lesion with one feeding pulmonary artery and one draining pulmonary vein. A complex pulmonary AVM is supplied by more than one feeder. A diffuse pulmonary AVM is a rare form of complex type and often involves all segments or, less commonly, the entire lobe [3].
Multidetector CT is currently considered the most diagnostic and least invasive examination method [12]. The classic appearance of pulmonary AVM is a well-defined peripheral nodule, which may be rounded or multilobulated. The pulmonary artery feeds into the nodule, and there are one or more draining veins, which are typically 1–2 mm larger than the feeding arteries (Fig. 2). On postcontrast CT during the pulmonary arterial phase, AVM appears as a homogeneously enhancing dilated vascular structure isoattenuating to the feeding and draining vessels (Fig. 2B). Maximum intensity projection or volume rendering images can help both outline the complex vascular anatomy of pulmonary vascular diseases and differentiate between them [8].
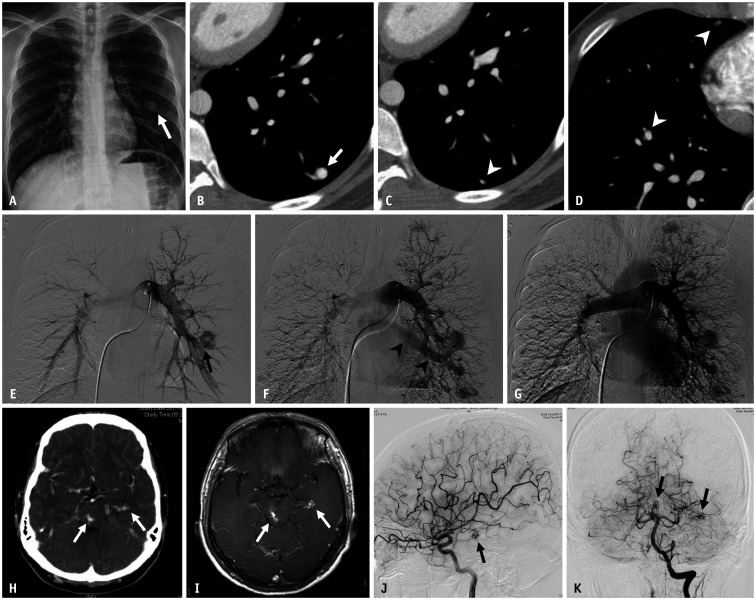
Fig. 2. A 19-year-old male patient with hereditary hemorrhagic telangiectasia.
A. Chest radiograph shows a well-circumscribed round opacity (arrow) in the lower third of the left hemithorax. B-D. Axial enhanced chest CT images demonstrate large arteriovenous malformation (arrow) with a feeding artery in the left lower lobe. Numerous, small arteriovenous malformations (arrowheads) are also identified in both lung fields. E, F. Arterial-phase images from the pulmonary angiogram reveal the feeding artery (arrow) and rapid opacification of the draining vein (arrowheads). G. Delayed venous-phase image shows opacification of the normal pulmonary veins. H, I. Contrast-enhanced brain CT and contrast-enhanced T1-weighted MR image demonstrate two small enhancing lesions (arrows) located at the left lateral ventricle temporal horn and midbrain, which suggest brain arteriovenous malformation. J, K. Left cerebral and vertebral angiograms confirm two small arteriovenous malformations (arrows).
Pulmonary angiography can intuitively delineate details of the angioarchitecture of pulmonary AVMs, such as the feeding pulmonary artery, sac, and draining veins, and is usually performed for therapeutic purposes. However, in some cases where connections between the pulmonary artery and vein are not clear on CT images, pulmonary angiography is considered for both diagnostic and therapeutic purposes [3]. On the angiogram, the feeder(s), sac, and draining vein were usually opacified almost at the same time (Supplementary Fig. 3). As the feeder(s) come closer to the nidus, it tends to become angulated and tortuous. The shape and size of the sac varied widely. In contrast, the drainage vein runs straight from the sac to the segmental pulmonary vein or, less commonly, the left atrium. The diameter of the draining veins was larger than that of the feeders. In delayed venous phases, the pulmonary veins from the segments or lobes other than those where the pulmonary AVM is located are opacified.
Transcatheter embolization is indicated when any of the following criteria are met: 1) any technically feasible pulmonary AVM with a feeding artery diameter as low as 2 mm, 2) measurable increase in the size of the pulmonary AVM, or 3) paradoxical emboli or symptomatic hypoxemia [13,14,15,16]. Various embolic materials, including vascular coils and plugs, are used to embolize the feeding arteries and/or sacs. The most common post-procedural complication is pleuritic chest pain, which typically occurs within two days, and is usually self-resolving [16]. There are a few rare but serious complications, including thrombus dislodging, vessel injury, or non-target embolization of embolic materials down to the heart [17]. Successful embolization was defined as a complete resolution or decrease in the size of the drainage vein by at least 70% (Supplementary Fig. 3D) on follow-up CT. Reperfusion or new pulmonary AVM can be seen in up to 20% of treated patients, which is generally suitable for repeat embolization [18].
Extrapulmonary AVM
Pleural or mediastinal AVM is a rare subset of high-flow congenital vascular malformations in which the nidus is located in the extrapulmonary spaces. The prevalence remains unknown, and the cause is either congenital in origin or acquired by inflammation, trauma, or neoplasm [19]. Most patients are asymptomatic and diagnosed incidentally during routine medical checkups. A few cases were associated with a pulsating bulging mass in the chest wall. However, radiologic follow-up is important because enlarged AVMs may encroach or invade vital mediastinal structures, such as the trachea or great vessels [20]. Severe hemorrhage from spontaneous rupture is rare, but can be a life-threatening complication. While pulmonary AVM is a well-known etiology of stroke through right-to-left shunt, pleural or mediastinal AVM rarely causes paradoxical embolization. The latter often has systemic veins as major draining channels, which means that a right-to-left shunt is less likely to occur [21].
Multidetector CT or MRI can be helpful in the differential diagnosis of extrapulmonary AVM and determining its relationship with the surrounding structures. Pleural or mediastinal AVM, which presents as a peripherally located vascular tangle abutting the pleural surface, may also mimic subpleural pulmonary AVM (Figs. 3, 4). It can have multiple systemic feeding arteries, including the intercostal, internal mammary, lateral thoracic, or phrenic arteries, and draining veins such as the intercostal, subclavian, or innominate veins [22]. These systemic arteries may be confused with systemic arterial supply to the lung in pulmonary sequestration, aberrant systemic artery supply without sequestration, or hypertrophic systemic artery due to lung disease (Supplementary Fig. 4) [23].
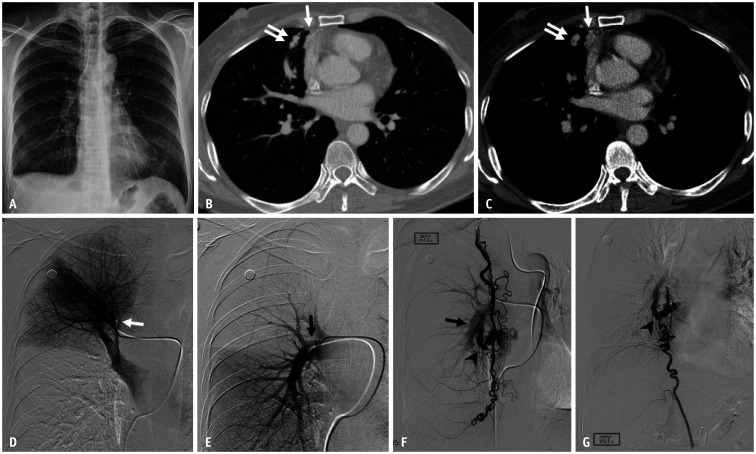
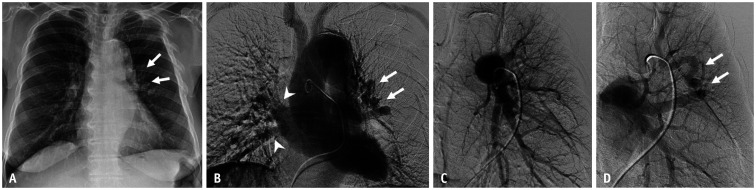
Fig. 3. A 49-year-old female patient with hereditary hemorrhagic telangiectasia who had a mediastinal arteriovenous malformation.
A. Chest radiograph shows no definite abnormality. B, C. Axial contrast-enhanced CT images with lung and mediastinal window settings demonstrate prominent draining veins (double arrows) at the periphery of the right middle lobe and along the mediastinal pleura (arrows). D, E. Selective angiograms of the truncus anterior (white arrow) and right interlobar pulmonary artery (black arrow) show no discernible vascular abnormalities. F, G. Feeding arteries originate from the hypertrophied right internal mammary and inferior phrenic arteries. Note also the large draining vein (arrowheads), which communicates with the right pulmonary artery (arrow). A nidus is seen at the beginning of the draining veins.
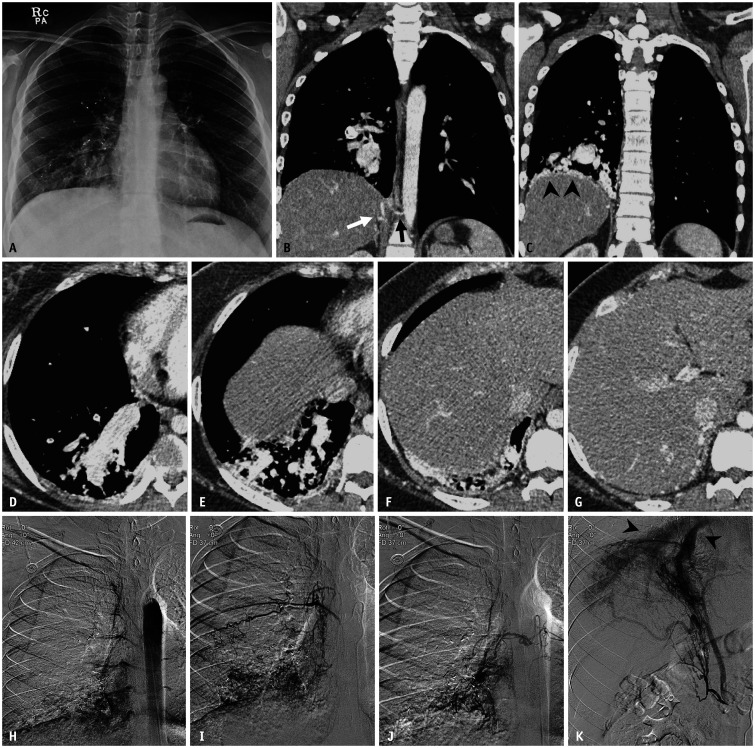
Fig. 4. A 19-year-old female patient with pleural arteriovenous malformation who underwent pulmonary artery embolization six years earlier.
A. Chest radiograph shows markedly enlarged vascular markings at the right lower lung zone. Multiple plugs from prior embolization procedures were also noted. B-G. Axial and coronal contrast-enhanced CT images with a mediastinal window setting demonstrate engorged vascular structures along the diaphragmatic pleural surface (arrowheads) and extremely dilated right inferior pulmonary veins. The feeding arteries originated from the hypertrophied right intercostal (black arrow) and inferior phrenic arteries (white arrow). H-K. Thoracic aortogram and selective angiograms of the right intercostal and inferior phrenic arteries reveal pleural arteriovenous malformation and early drainage through dilated right inferior pulmonary veins (arrowheads).
Although pulmonary vessels can be a feeding artery or draining vein for pleural or mediastinal AVM, when pulmonary angiography fails to demonstrate either feeders or nidus or drainage veins, systemic feeders should be investigated. Therefore, when mediastinal AVM is on the differential diagnosis list, both the femoral arterial and venous approaches should be used for diagnostic angiography. The treatment strategy for extrapulmonary AVM is different from that for pulmonary AVM. Embolization of the feeders is the mainstay of pulmonary AVM treatment. However, sclerotherapy of the nidus is a key factor in the treatment of pleural or mediastinal AVMs [24].
Pulmonary Venous Anomalies
Anomalous Pulmonary Vein
An anomalous pulmonary vein indicates that pulmonary venous drainage of the affected lung is disturbed and instead occurs through the normal pulmonary vein via collateral veins, which can even traverse the fissure or lobes. It may occur in association with other congenital anomalies, such as pulmonary vein atresia or stenosis. The anomalous pulmonary vein is most frequently misdiagnosed as pulmonary AVM because the dilated and tortuous anomalous collateral draining channel is similar to the dilated draining vein from pulmonary AVM on CT [5]. From this perspective, an anomalous pulmonary vein has a normal capillary network with no nidus and, therefore, can be followed up without treatment. Patients are usually asymptomatic but can have dyspnea, respiratory distress, or hemoptysis.
An anomalous pulmonary vein can involve the entire unilateral lung or lobe(s), segment, or subsegment. Accordingly, its nomenclature has been confusing in the literature [5,25]. The most well-known one is the anomalous unilateral single pulmonary vein, which is defined as a single pulmonary vein draining the entire ipsilateral lung (Fig. 5). In contrast, if an anomalous pulmonary vein involves only part of the lung, more than one pulmonary vein can drain the ipsilateral lung (Figs. 6, 7, 8, Supplementary Fig. 5) [26].
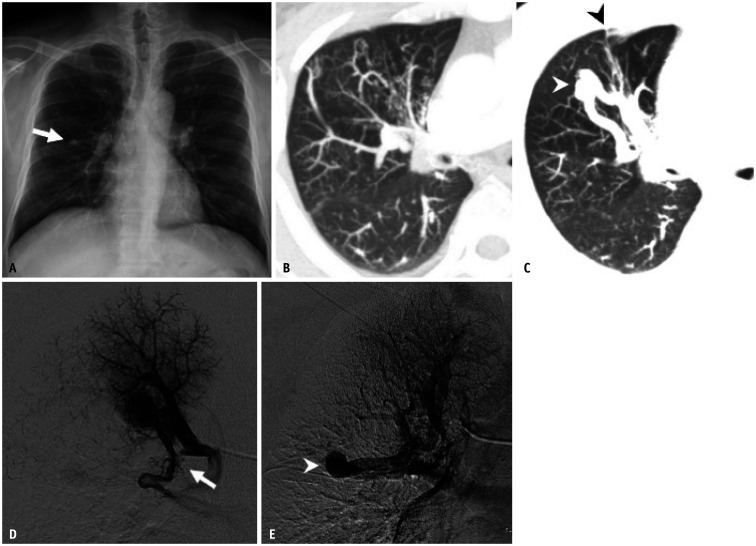
Fig. 5. An anomalous pulmonary vein caused by lobar pulmonary vein atresia in a 32-year-old male patient.
Adapted from Lee et al. Korean J Radiol 2011;12:395-399 [30].
A. Serial axial CT images show a low attenuation linear structure (arrows) between the right inferior pulmonary vein and left atrium, as well as a prominent right superior pulmonary vein(s). An abnormally enlarged vascular structure (double arrow) near the right pulmonary artery, suggesting the presence of collateral drainage of the interrupted inferior pulmonary vein, was also noted. B. The reformatted oblique sagittal CT image shows the atretic right inferior pulmonary vein (arrow). C. The reformatted coronal image with the lung setting reveals multiple tortuous and dot-like collaterals (arrowheads) in the superior segment of the right lower lobe crossing the major fissure. D-F. Venous-phase images of the right interlobar selective pulmonary angiogram show no connection between the left atrium and the right inferior pulmonary vein (double arrowhead), which is draining into the superior pulmonary vein (arrowhead) through the collaterals.
Fig. 6. An anomalous pulmonary vein in a 75-year-old female patient who presented with persistent blood-tinged sputum.
Adapted from Hyun et al. Cardiovasc Intervent Radiol 2014;37:835-838, with permission of the Springer Nature [4].
A. Chest radiographs show a tortuous abnormal vascular structure (arrows) at the left parahilar area. B. Venous-phase image in the main pulmonary angiogram shows a tortuous and dilated abnormal vessel in the left parahilar area, which drains into the left atrium through an anomalous pulmonary vein (arrows). The right superior and inferior pulmonary veins drain normally into the left atrium (arrowheads). C. Early arterial-phase image of the left upper lobar selective pulmonary angiogram shows no early draining vein. D. An anomalous venous structure (arrows) is seen in the late-venous phase.
Fig. 7. An anomalous pulmonary vein and segmental pulmonary vein atresia in a 43-year-old male patient who presented with persistent cough and sputum production.
Adapted from Lee et al. Korean J Radiol 2011;12:395-399 [30].
A. Chest radiograph shows a single nodular opacity (arrow) in the right mid-lung zone. B, C. Slab axial images with the lung window setting show tortuous and dot-like collaterals and an aneurysmal dilatation of vascular structure (white arrowhead) crossing the minor fissure (black arrowhead) in the anterior segment of the right upper lobe. D, E. Venous-phase images in the right apical segmental selective pulmonary angiogram show that the apical segmental pulmonary vein is interrupted (arrow). Venous drainage of the affected segment detours via pulmonary vein varix associated with anomalous pulmonary vein (arrowhead).
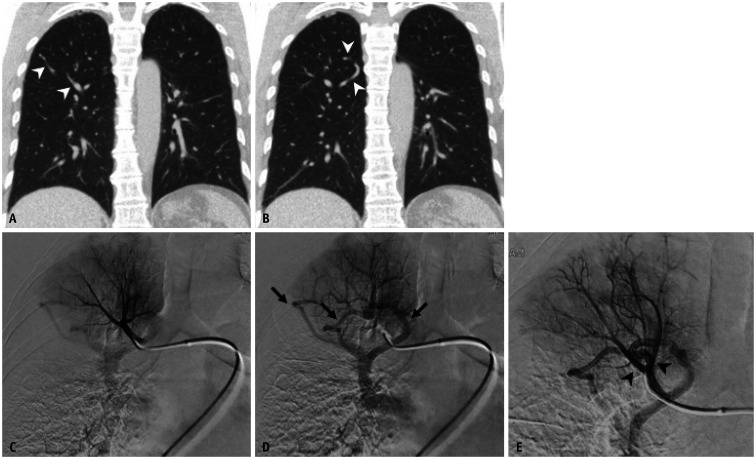
Fig. 8. A 61-year-old female with an anomalous pulmonary vein at the segmental level.
A, B. Coronal CT images demonstrate several tortuous vascular structures (arrowheads) in the right upper lobe. C, D. Selective angiograms at the truncus anterior show normal pulmonary arteries, but abnormal drainage of the right upper lobe apical segment (arrows) through anomalous pulmonary veins. E. In the angiogram with a catheter wedged into the segmental pulmonary artery, the anomalous pulmonary vein mimics a nidus and the early draining vein (arrowheads) of arteriovenous malformation.
Anomalous pulmonary veins are usually found incidentally on chest radiographs or cross-sectional images. Chest radiographs may show a non-specific tubular or oval shadow (Fig. 6A), a parenchymal nodule (Fig. 7A), or a pericardial curvilinear shadow. Multidetector CT angiography is currently considered the reference modality for diagnosing pulmonary venous anomalies, which enables a clear depiction of the anomalous vascular structure.
Several studies have described the CT features of anomalous pulmonary veins to help distinguish anomalous pulmonary veins from pulmonary AVMs [1,2,5]. They include 1) an anomalous pulmonary vein, which may imitate a tangled vascular structure (sac), but the feeding artery and relevant pulmonary vein are absent, and 2) an anomalous pulmonary vein that courses through the lung lobes, often crossing the fissure if it develops before pulmonary segmentation (Fig. 7C).
Selective pulmonary angiography, although invasive, could play an important role in the differential diagnosis of an anomalous pulmonary vein when the CT findings are equivocal [4,5,25,27]. In contrast to a pulmonary AVM, an anomalous pulmonary vein has no feeding artery or nidus. An interrupted or severely stenosed pulmonary vein is visible on delayed-phase images, and the diameter can be larger than that of a normal pulmonary vein (Fig. 7D-E). Pulmonary venous return from the affected lung parenchyma occurs via dilated and tortuous collateral channels that should not be misinterpreted as the nidus and draining vein of the pulmonary AVM. These collateral channels finally meet any of the pulmonary veins that are normally connected to the left atrium. These findings become clearer when compared to pulmonary angiograms of the contralateral lung. However, when pulmonary angiography is performed with the angiographic catheter wedge, the findings can become confusing (Fig. 8E).
Pulmonary Vein Atresia and Stenosis
Pulmonary vein atresia or stenosis is a rare but serious disease that may lead to progressive pulmonary hypertension and eventually heart failure [28]. The congenital cause is the abnormal incorporation of the pulmonary vein into the left atrium (Supplementary Fig. 6). It may occur as an isolated anomaly or in association with other congenital cardiac anomalies. The acquired causes include complications of previous surgical repairs for cardiac anomalies, radiofrequency ablation for arrhythmia, and less frequently, neoplasm or inflammation [8]. The severity of the symptoms depends largely on the number of pulmonary veins involved and the severity of the obstruction [29].
Pulmonary vein atresia or stenosis may involve one or more pulmonary veins and can be divided into common, unilateral, and individual types. The individual type involves the lobar or segmental pulmonary vein and has been suggested as a possible cause of an anomalous pulmonary vein (Figs. 5, 7) [30]. On CT or pulmonary angiography, venous drainage from the affected lung drains to one of the normal pulmonary veins through various forms of collaterals from multiple fine channels to a dilated tortuous vascular structure (Fig. 5C).
Symptomatic patients with pulmonary vein atresia usually require surgical treatment, and percutaneous intervention is the treatment of choice for patients with pulmonary vein stenosis. However, published studies reporting the treatment outcomes of pulmonary vein atresia or stenosis demonstrated that neither surgical nor endovascular intervention achieved satisfactory long-term outcomes, with high rates of restenosis, reintervention, and mortality [31]. In recent clinical trials, various treatment approaches have been attempted with drug-eluting stents, chemotherapeutic agents, and molecular target agents to prevent restenosis [32].
Pulmonary Vein Varix
Pulmonary vein varix is an aneurysmal dilatation of the pulmonary vein, also known as a pulmonary venous aneurysm. The size criteria for a pulmonary vein varix have not been established, but an aneurysm is usually defined as a 50% increase from the normal diameter [33]. The etiology of a pulmonary vein varix remains uncertain but can be congenital or acquired. Congenital varices may be in the context of an anomalous pulmonary venous development, such as pulmonary vein atresia and anomalous pulmonary vein, leading to increased blood flow and dilatation with the Laplace’s law (Fig. 7, Supplementary Fig. 7) [34]. Acquired varices are associated with diseases of increased pulmonary vein pressure, such as mitral valve disease, liver cirrhosis, or emphysema.
The angiographic diagnostic criteria for confirmative diagnosis have been suggested as follows [35]: 1) an anomalous dilated perihilar pulmonary vein, sparing the peripheral region, which fills at the same rate as the normal pulmonary veins, 2) no evidence of pulmonary arterial dilatation or arteriovenous fistula (Supplementary Fig. 7E), and 3) the drainage from the anomalous pulmonary vein to the left atrium is delayed compared to other normal pulmonary veins (Supplementary Fig. 7G).
The differential diagnosis from other vascular anomalies is important because a pulmonary vein varix is usually asymptomatic and does not require treatment once the correct diagnosis is established. Acquired varices secondary to pulmonary venous hypertension generally regress with correction of the underlying cause. However, annual monitoring with imaging studies should be considered to evaluate the possible complications [36]. For patients with pulmonary varices complicated by enlargement on imaging, hemoptysis or thromboembolism are candidates for surgical intervention [34].
Partial Anomalous Pulmonary Venous Drainage
Partial anomalous pulmonary venous drainage (PAPVD) describes the connection of at least one pulmonary vein, but not all, to the systemic venous system or the right atrium. The drainage of all pulmonary veins outside the left atrium is termed total anomalous pulmonary venous drainage (TAPVD). The reported prevalence of PAPVD has been reported to be between 0.4% and 0.7% [6]. While patients with TAPVD are usually symptomatic and require surgical repair during the infant period, most PAPVD cases are detected incidentally. If anomalous venous drainage compromises 50% or more, it may become clinically significant and manage treatment.
The most common type of PAPVD is the involvement of the right superior pulmonary vein, which frequently occurs at the junction of the superior vena cava and right atrium (Supplementary Fig. 8) [37]. This subset is associated with a superior sinus venosus atrial septal defect near the orifice of the superior vena cava. Left-sided PAPVD commonly affected the left superior pulmonary vein (Fig. 9). The affected vein typically courses lateral to the aortic arch prior to draining into the left brachiocephalic vein, which can often be misdiagnosed as the left superior vena cava (Supplementary Fig. 9). Other described anomalous connections include drainage into the azygos vein, hemiazygos vein, coronary sinus, or inferior vena cava.
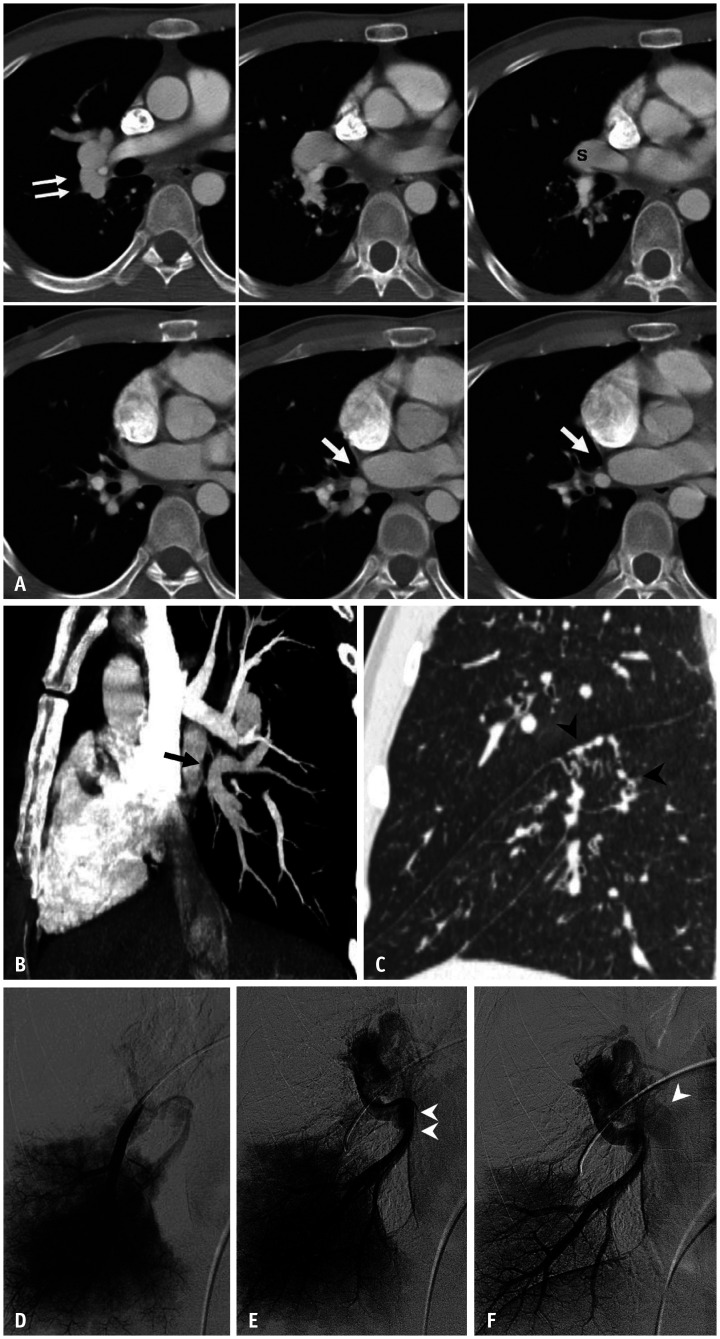
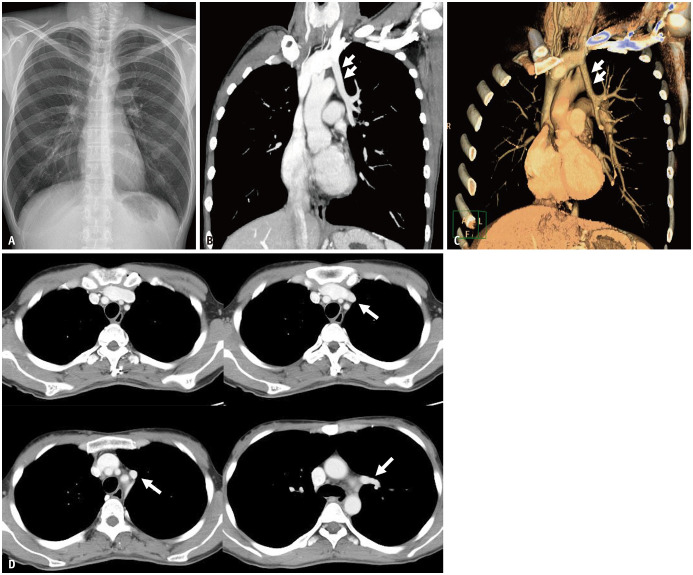
Fig. 9. Partial anomalous pulmonary venous drainage involving the left superior pulmonary vein in a 38-year-old female.
A. Chest radiograph shows no definite abnormality. B, C. Coronal CT image and volume-rendered image demonstrate that a vertical anomalous left superior pulmonary vein (arrows) courses lateral to the aortic arch prior to draining into the left brachiocephalic vein. D. Serial axial CT images show that the anomalous left superior pulmonary vein (arrows) drains into the left brachiocephalic vein.
Scimitar syndrome, a unique subset of PAPVD, consists of right-sided anomalous pulmonary venous drainage, hypoplasia of the lung, cardiac dextroposition, and systemic arterial supply to the right lower lobe [38]. The scimitar vein usually drains the entire right lung, courses anterior to the hilum, and enter the subdiaphragmatic IVC. The typical radiologic finding is a scimitar sign, a curved opacity lateral to the right cardiac border, which extends toward the inferior vena cava (Supplementary Fig. 10). A scimitar variant, also known as a pseudo-scimitar syndrome, is a condition in which the anomalous pulmonary vein course is similar to that of the scimitar sign but has a normal connection to the left atrium or has dual connections to the inferior vena cava and left atrium [39,40].
Contrast-enhanced CT clearly demonstrates the anatomic details of anomalous pulmonary venous drainage in most cases. Conventional angiography is not essential for diagnosis but can play a significant role in the treatment process. Affected patients with significant left-to-right shunts have traditionally been considered candidates for surgical correction. A thorough delineation of the anatomy of individual patients is important for surgical planning. Several cases of percutaneous PAPVD embolization to reduce the amount of shunt flow have also been reported (Supplementary Fig. 11) [41,42]. In selected patients with favorable anatomy, percutaneous treatment should be considered before surgical management.
Pulmonary Arterial Anomalies
Pulmonary Artery Aneurysm or Ectasia
True aneurysm of the pulmonary artery is rare and may be congenital or acquired in origin (Fig. 10). It has a variety of preceding factors, including Eisenmenger syndrome, pulmonary valve disease, pulmonary hypertension, autoimmune disease, and vasculitis [43]. There are often complex interactions between several factors that lead to aneurysm formation. False aneurysms (pseudoaneurysms) of the pulmonary artery are predominantly acquired and are caused by various diagnostic or therapeutic procedures, trauma, and infectious or malignant disease (Fig. 11) [44]. In contrast, pulmonary artery ectasia refers to diffuse dilatation of the pulmonary arteries without focal aneurysmal dilatation (Fig. 12).
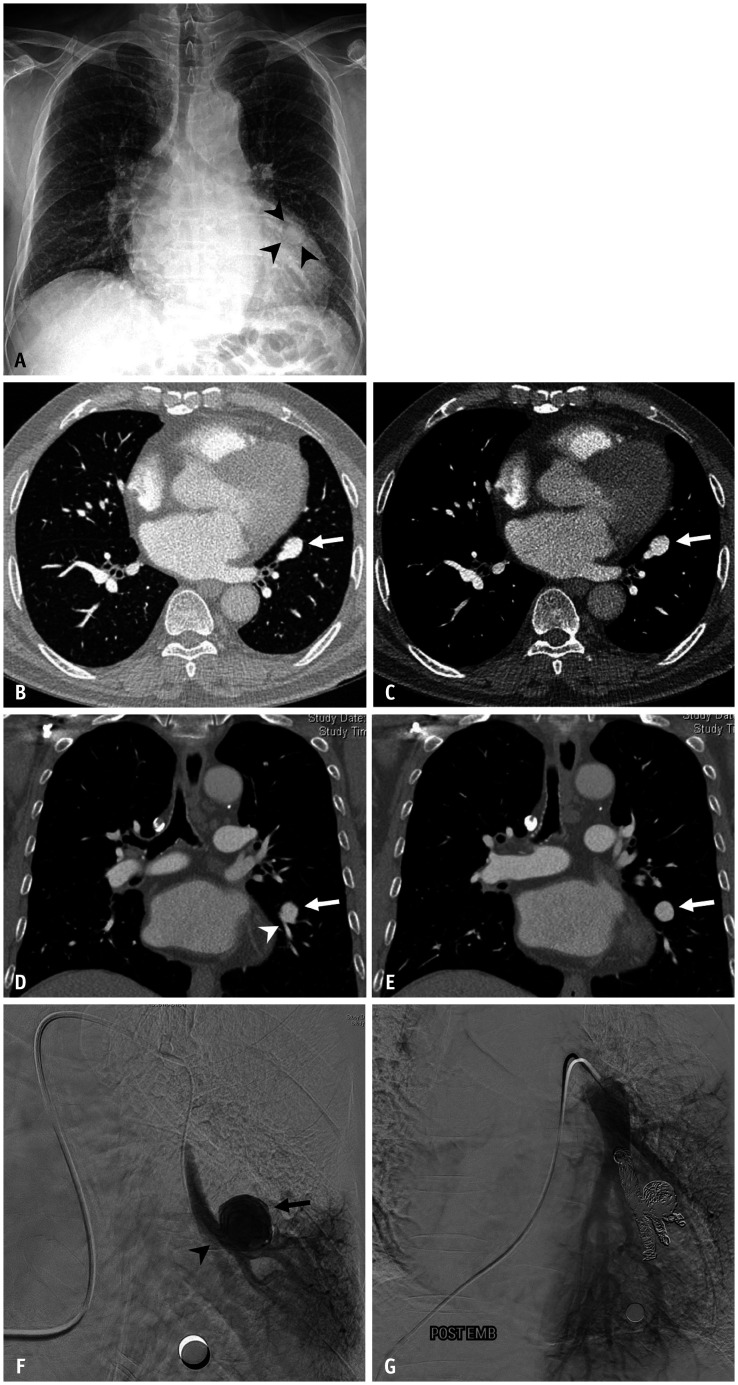
Fig. 10. Pulmonary artery aneurysm in a 68-year-old male.
A. Chest radiograph shows a rounded shadow (arrowheads) superimposed onto the enlarged cardiac silhouette. B-E. Enhanced CT images in the lung and mediastinal window settings reveal an aneurysm (arrows) arising from the left lower lobe segmental pulmonary artery (arrowhead). F. Digital subtraction angiography image with the catheter tip in the aneurysmal sac (arrow) shows a segmental branch of the left lower lobe pulmonary artery (arrowhead), but no draining veins. G. Post-embolization angiogram depicts complete packing of the segmental pulmonary artery proximal and distal to the aneurysm with coils.
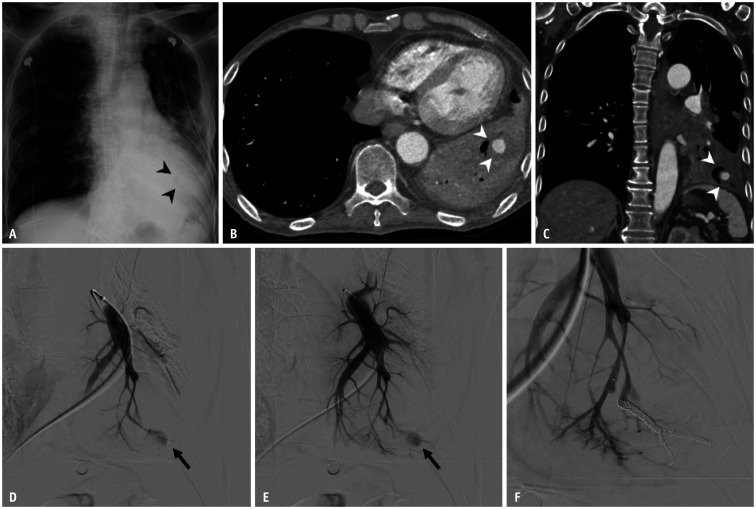
Fig. 11. Pulmonary artery pseudoaneurysm in a 65-year-old male with lung cancer undergoing chemotherapy.
A. Chest radiograph shows a thin, radiolucent crescent-shaped structure (arrowheads) superimposed onto the enlarged cardiac silhouette. B, C. Axial and coronal CT images reveal an air crescent adjacent to a pseudoaneurysm (arrowheads) with surrounding consolidation. D, E. Selective angiogram of the left lower lobe pulmonary artery demonstrates the pseudoaneurysm (arrows) arising from the segmental branch. F. Completion angiogram after coil embolization shows the absence of pseudoaneurysm filling.
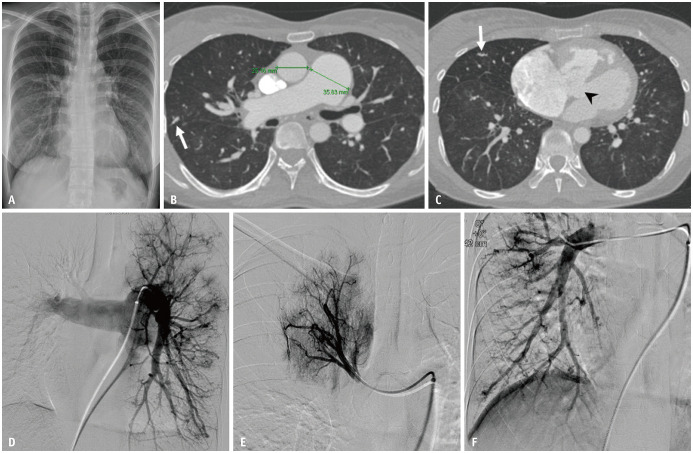
Fig. 12. Pulmonary artery ectasia in a 56-year-old female patient with hemoptysis.
A. Chest radiographs show mild cardiomegaly and prominent bilateral pulmonary vascular markings. B, C. Axial images in the lung window setting show dilatation of the pulmonary trunk to a maximum diameter of 36 mm with dilated and beaded peripheral pulmonary arteries (arrows). A ventricular septal defect is also noted (arrowhead). D-F. Arterial-phase images of the selective bilateral pulmonary angiogram reveal diffuse dilatation of the pulmonary trunk and branches.
Imaging features can vary depending on etiology. Pulmonary artery aneurysm associated with a congenital cause and pulmonary hypertension tends to be localized to the main pulmonary artery and/or branches. Aneurysms associated with autoimmune disease and vasculitis tend to be multiple, with bilateral involvement of the distal pulmonary circulation. Pseudoaneurysms associated with tuberculosis are referred to as Rasmussen aneurysms and usually involve the upper lobes in a setting of tuberculosis reactivation. On CT or conventional angiography, they can be confused with pulmonary AVM if peripherally located and without any underlying cause [12]. However, a pulmonary artery aneurysm is not associated with early opacification of the draining vein, which is a characteristic of pulmonary AVM.
Differential diagnosis is important for determining an appropriate treatment plan. The treatment of a pulmonary artery aneurysm must first be tailored to manage underlying conditions such as pulmonary hypertension and vasculitis [43]. Surgical intervention is indicated if the size increases or if the aneurysm becomes symptomatic. Alternatively, a peripheral pulmonary artery aneurysm can be treated with endovascular embolization where pulmonary function and adequate reserve are available, similar to a pulmonary AVM [45].
CONCLUSION
Various vascular diseases involving the lung, pleura, and mediastinum can be confused with pulmonary AVM in terms of clinical presentation and radiologic findings. These include extrapulmonary AVM, an anomalous pulmonary vein, pulmonary vein atresia or stenosis, PAPVD, pulmonary vein varix, and pulmonary artery aneurysms. Each of them has unique radiologic features and can be differentiated through careful analysis of contrast-enhanced CT images in most cases. However, when the cross-sectional imaging findings are inconclusive, angiography of the pulmonary arteries and even systemic arteries is considered. Therefore, interventional radiologists need to be aware of the angiographic features of pulmonary AVM and its mimickers to help establish a correct diagnosis and offer proper management strategies.
Acknowledgments
Authors deeply appreciate Prof. Young Tong Kim from Soonchunhyang University Cheonan Hospital and Prof. Ji Hoon Shin from Asan Medical Center for their contribution on valuable cases.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: all authors.
- Project administration: all authors.
- Resources: all authors.
- Supervision: Dongho Hyun.
- Writing—original draft: Hyoung Nam Lee.
- Writing—review & editing: all authors.
Funding Statement: This work was supported by the Soonchunhyang University Research Fund.
Availability of Data and Material
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2021.0417
References
- 1.Hanson JM, Wood AM, Seymour R, Petheram IS. Anomalous unilateral single pulmonary vein: two cases mimicking arteriovenous malformations and a review of the literature. Australas Radiol. 2005;49:246–251. doi: 10.1111/j.1440-1673.2005.01423.x. [DOI] [PubMed] [Google Scholar]
- 2.Tokunaga K, Kubo T, Yamaoka T, Isoda H, Togashi K. Can the “pine-needle sign” on computed tomography be used to differentiate pulmonary arteriovenous malformation from its mimics? Analysis based on dynamic contrast-enhanced chest computed tomography in adults. Eur J Radiol. 2017;95:314–318. doi: 10.1016/j.ejrad.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 3.Gill SS, Roddie ME, Shovlin CL, Jackson JE. Pulmonary arteriovenous malformations and their mimics. Clin Radiol. 2015;70:96–110. doi: 10.1016/j.crad.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Hyun D, Do YS, Lim SJ, Park HS, Park KB. Anomalous unilateral single pulmonary vein mimicking pulmonary arteriovenous malformation. Cardiovasc Intervent Radiol. 2014;37:835–838. doi: 10.1007/s00270-013-0723-y. [DOI] [PubMed] [Google Scholar]
- 5.Lin CT, Zimmerman SL, Mitchell SE, Fishman EK. Pulmonary venous anomalies causing misdiagnosis of pulmonary arteriovenous malformations. Clin Imaging. 2018;47:96–100. doi: 10.1016/j.clinimag.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Lyen S, Wijesuriya S, Ngan-Soo E, Mathias H, Yeong M, Hamilton M, et al. Anomalous pulmonary venous drainage: a pictorial essay with a CT focus. J Congenit Heart Dis. 2017;1:7 [Google Scholar]
- 7.Bueno J, Flors L, Mejía M. Congenital anomalies of the pulmonary arteries: spectrum of findings on computed tomography. Radiologia. 2017;59:209–217. doi: 10.1016/j.rx.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Porres DV, Morenza OP, Pallisa E, Roque A, Andreu J, Martínez M. Learning from the pulmonary veins. Radiographics. 2013;33:999–1022. doi: 10.1148/rg.334125043. [DOI] [PubMed] [Google Scholar]
- 9.Castañer E, Gallardo X, Rimola J, Pallardó Y, Mata JM, Perendreu J, et al. Congenital and acquired pulmonary artery anomalies in the adult: radiologic overview. Radiographics. 2006;26:349–371. doi: 10.1148/rg.262055092. [DOI] [PubMed] [Google Scholar]
- 10.Gossage JR, Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med. 1998;158:643–661. doi: 10.1164/ajrccm.158.2.9711041. [DOI] [PubMed] [Google Scholar]
- 11.Mu W, Cordner ZA, Yuqi Wang K, Reed K, Robinson G, Mitchell S, et al. Characterization of pulmonary arteriovenous malformations in ACVRL1 versus ENG mutation carriers in hereditary hemorrhagic telangiectasia. Genet Med. 2018;20:639–644. doi: 10.1038/gim.2017.160. [DOI] [PubMed] [Google Scholar]
- 12.Saboo SS, Chamarthy M, Bhalla S, Park H, Sutphin P, Kay F, et al. Pulmonary arteriovenous malformations: diagnosis. Cardiovasc Diagn Ther. 2018;8:325–337. doi: 10.21037/cdt.2018.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller-Hülsbeck S, Marques L, Maleux G, Osuga K, Pelage JP, Wohlgemuth WA, et al. CIRSE standards of practice on diagnosis and treatment of pulmonary arteriovenous malformations. Cardiovasc Intervent Radiol. 2020;43:353–361. doi: 10.1007/s00270-019-02396-2. [DOI] [PubMed] [Google Scholar]
- 14.Shovlin CL, Condliffe R, Donaldson JW, Kiely DG, Wort SJ British Thoracic Society. British Thoracic Society clinical statement on pulmonary arteriovenous malformations. Thorax. 2017;72:1154–1163. doi: 10.1136/thoraxjnl-2017-210764. [DOI] [PubMed] [Google Scholar]
- 15.Faughnan ME, Palda VA, Garcia-Tsao G, Geisthoff UW, McDonald J, Proctor DD, et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet. 2011;48:73–87. doi: 10.1136/jmg.2009.069013. [DOI] [PubMed] [Google Scholar]
- 16.Chamarthy MR, Park H, Sutphin P, Kumar G, Lamus D, Saboo S, et al. Pulmonary arteriovenous malformations: endovascular therapy. Cardiovasc Diagn Ther. 2018;8:338–349. doi: 10.21037/cdt.2017.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawale A, Chealikani G, Mitchell L, Clark S. Imaging modalities for retrieval of a migrated coil from the left ventricle, after pulmonary arterio-venous malformation embolisation. Interact Cardiovasc Thorac Surg. 2009;9:543–544. doi: 10.1510/icvts.2008.199471. [DOI] [PubMed] [Google Scholar]
- 18.Milic A, Chan RP, Cohen JH, Faughnan ME. Reperfusion of pulmonary arteriovenous malformations after embolotherapy. J Vasc Interv Radiol. 2005;16:1675–1683. doi: 10.1097/01.RVI.0000182163.25493.BB. [DOI] [PubMed] [Google Scholar]
- 19.Bonne L, Vanhoenacker P, Defreyne L. Embolization of a giant intercostal aneurysm and arteriovenous malformation. Cardiovasc Intervent Radiol. 2015;38:1352–1355. doi: 10.1007/s00270-014-1007-x. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan T, Altuntas B, Ceran S, Sadi Sunam G. Unusual location of arteriovenous malformation; posterior mediastinum. Interact Cardiovasc Thorac Surg. 2009;8:260–262. doi: 10.1510/icvts.2008.195198. [DOI] [PubMed] [Google Scholar]
- 21.Rajiah P, Kanne JP. Mediastinal vascular malformation presenting with stroke. Br J Radiol. 2010;83:e138–e142. doi: 10.1259/bjr/68475678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tennyson C, Routledge T, Chambers A, Scarci M. Arteriovenous malformation in the anterior mediastinum. Ann Thorac Surg. 2010;90:e9–e10. doi: 10.1016/j.athoracsur.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 23.Do KH, Goo JM, Im JG, Kim KW, Chung JW, Park JH. Systemic arterial supply to the lungs in adults: spiral CT findings. Radiographics. 2001;21:387–402. doi: 10.1148/radiographics.21.2.g01mr06387. [DOI] [PubMed] [Google Scholar]
- 24.Kim YW, Lee BB, Yakes WF, Do YS. Congenital vascular malformations: a comprehensive review of current management. Berlin: Springer; 2017. [Google Scholar]
- 25.Mataciunas M, Gumbiene L, Tamosiunas A, Laucevicius A. Meandering pulmonary veins mimicking arteriovenous malformation. J Cardiovasc Comput Tomogr. 2015;9:149–150. doi: 10.1016/j.jcct.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues MA, Ritchie G, Murchison JT. Incidental meandering right pulmonary vein, literature review and proposed nomenclature revision. World J Radiol. 2013;5:215–219. doi: 10.4329/wjr.v5.i5.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasuo K, Numaguchi Y, Kishikawa T, Ikeda J, Matsuura K. Anomalous unilateral single pulmonary vein mimicking pulmonary varices. Chest. 1981;79:602–604. doi: 10.1378/chest.79.5.602. [DOI] [PubMed] [Google Scholar]
- 28.Cullen S, Deasy PF, Tempany E, Duff DF. Isolated pulmonary vein atresia. Br Heart J. 1990;63:350–354. doi: 10.1136/hrt.63.6.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latson LA, Prieto LR. Congenital and acquired pulmonary vein stenosis. Circulation. 2007;115:103–108. doi: 10.1161/CIRCULATIONAHA.106.646166. [DOI] [PubMed] [Google Scholar]
- 30.Lee HN, Kim YT, Cho SS. Individual pulmonary vein atresia in adults: report of two cases. Korean J Radiol. 2011;12:395–399. doi: 10.3348/kjr.2011.12.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balasubramanian S, Marshall AC, Gauvreau K, Peng LF, Nugent AW, Lock JE, et al. Outcomes after stent implantation for the treatment of congenital and postoperative pulmonary vein stenosis in children. Circ Cardiovasc Interv. 2012;5:109–117. doi: 10.1161/CIRCINTERVENTIONS.111.964189. [DOI] [PubMed] [Google Scholar]
- 32.Suntharos P, Prieto LR. Treatment of congenital and acquired pulmonary vein stenosis. Curr Cardiol Rep. 2020;22:153. doi: 10.1007/s11886-020-01395-x. [DOI] [PubMed] [Google Scholar]
- 33.Kim YH, Marom EM, Herndon JE, 2nd, McAdams HP. Pulmonary vein diameter, cross-sectional area, and shape: CT analysis. Radiology. 2005;235:43–49. doi: 10.1148/radiol.2351032106. discussion 49-50. [DOI] [PubMed] [Google Scholar]
- 34.Gleason JB, Shekar SP, Hernandez F, Valentin R, Mehta JP. Pulmonary varix: an uncommon pulmonary vascular anomaly. Clin Pulm Med. 2017;24:87–91. [Google Scholar]
- 35.Berecova Z, Neuschl V, Boruta P, Masura J, Ghersin E. A complex pulmonary vein varix -- diagnosis with ECG gated MDCT, MRI and invasive pulmonary angiography. J Radiol Case Rep. 2012;6:9–16. doi: 10.3941/jrcr.v6i12.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haddad J, Badran A, Pavão R, de Padua AI, Lago I, Marin Neto JA. Pulmonary varix: a case report. Rev Port Cardiol. 2016;35:443.e1–443.e4. doi: 10.1016/j.repc.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Pandey NN, Sharma A, Jagia P. Imaging of anomalous pulmonary venous connections by multidetector CT angiography using third-generation dual source CT scanner. Br J Radiol. 2018;91:20180298. doi: 10.1259/bjr.20180298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goudarzi M, Sabouri S, Fayazi N, Shabestari AA, Karam MB, Kahkouee S. Anomalous unilateral single pulmonary vein mimicking pulmonary nodules on HRCT of the lungs and scimitar syndrome on chest radiograph: multidetector CT findings. J Thorac Imaging. 2009;24:142–146. doi: 10.1097/RTI.0b013e318190424e. [DOI] [PubMed] [Google Scholar]
- 39.Odenthal C, Sarikwal A. Anomalous unilateral single pulmonary vein versus scimitar syndrome: comparison of two paediatric cases and a review of the literature. J Med Imaging Radiat Oncol. 2012;56:247–254. doi: 10.1111/j.1754-9485.2012.02385.x. [DOI] [PubMed] [Google Scholar]
- 40.Pearl W. Scimitar variant. Pediatr Cardiol. 1987;8:139–141. doi: 10.1007/BF02079472. [DOI] [PubMed] [Google Scholar]
- 41.Gangadhara MB, Magee AG. Transcatheter occlusion of partial anomalous pulmonary venous connection with dual drainage to left atrium. Ann Pediatr Cardiol. 2019;12:144–146. doi: 10.4103/apc.APC_72_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson W, Horlick E, Benson L. Successful transcatheter occlusion of an anomalous pulmonary vein with dual drainage to the left atrium. Catheter Cardiovasc Interv. 2015;85:1212–1216. doi: 10.1002/ccd.25734. [DOI] [PubMed] [Google Scholar]
- 43.Gupta M, Agrawal A, Iakovou A, Cohen S, Shah R, Talwar A. Pulmonary artery aneurysm: a review. Pulm Circ. 2020;10:2045894020908780. doi: 10.1177/2045894020908780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pelage JP, El Hajjam M, Lagrange C, Chinet T, Vieillard-Baron A, Chagnon S, et al. Pulmonary artery interventions: an overview. Radiographics. 2005;25:1653–1667. doi: 10.1148/rg.256055516. [DOI] [PubMed] [Google Scholar]
- 45.Park HS, Chamarthy MR, Lamus D, Saboo SS, Sutphin PD, Kalva SP. Pulmonary artery aneurysms: diagnosis & endovascular therapy. Cardiovasc Diagn Ther. 2018;8:350–361. doi: 10.21037/cdt.2018.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.