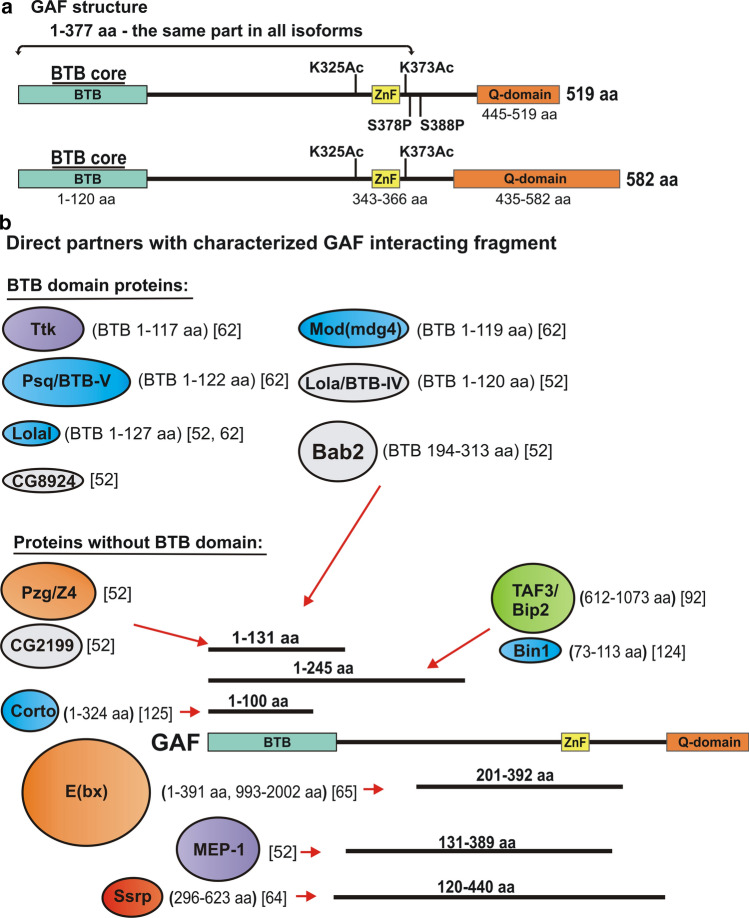
Fig. 1.
a Structure of GAF519 and GAF582 isoforms. Both isoforms share a 1-377 aa N-terminal domain that contains BTB/POZ domain and the C2H2-type zinc finger, while they have alternative C-terminal domains that are rich poly Q sequences. Amino acids K325 and K373 are targets for acetylation, while S378 and S388 may be phosphorylated in GAF519. b Proteins that interact directly with GAF. Proteins are shown as colored ovals. The size of the oval indicates the relative size of the proteins. The sequences for each protein that mediate interactions with GAF are indicated in the round brackets, while the corresponding references are indicated in the square brackets. Proteins that interact with GAF via their BTB domain are: Ttk, Mod(mdg4), Psq/BTB-V, Lola/BTB-IV, Lolal, Bab2, CG8924. Protein partners that interact with GAF but lack a BTB domain are: Pzq/Z4, CG2199, Corto, E(bx), Mep-1, Ssrp, TAF3/Bip2, Bin1. Red arrows indicate the GAF sequences that are required for interaction