Fig. 2. Lyn directly phosphorylates NLRP3.
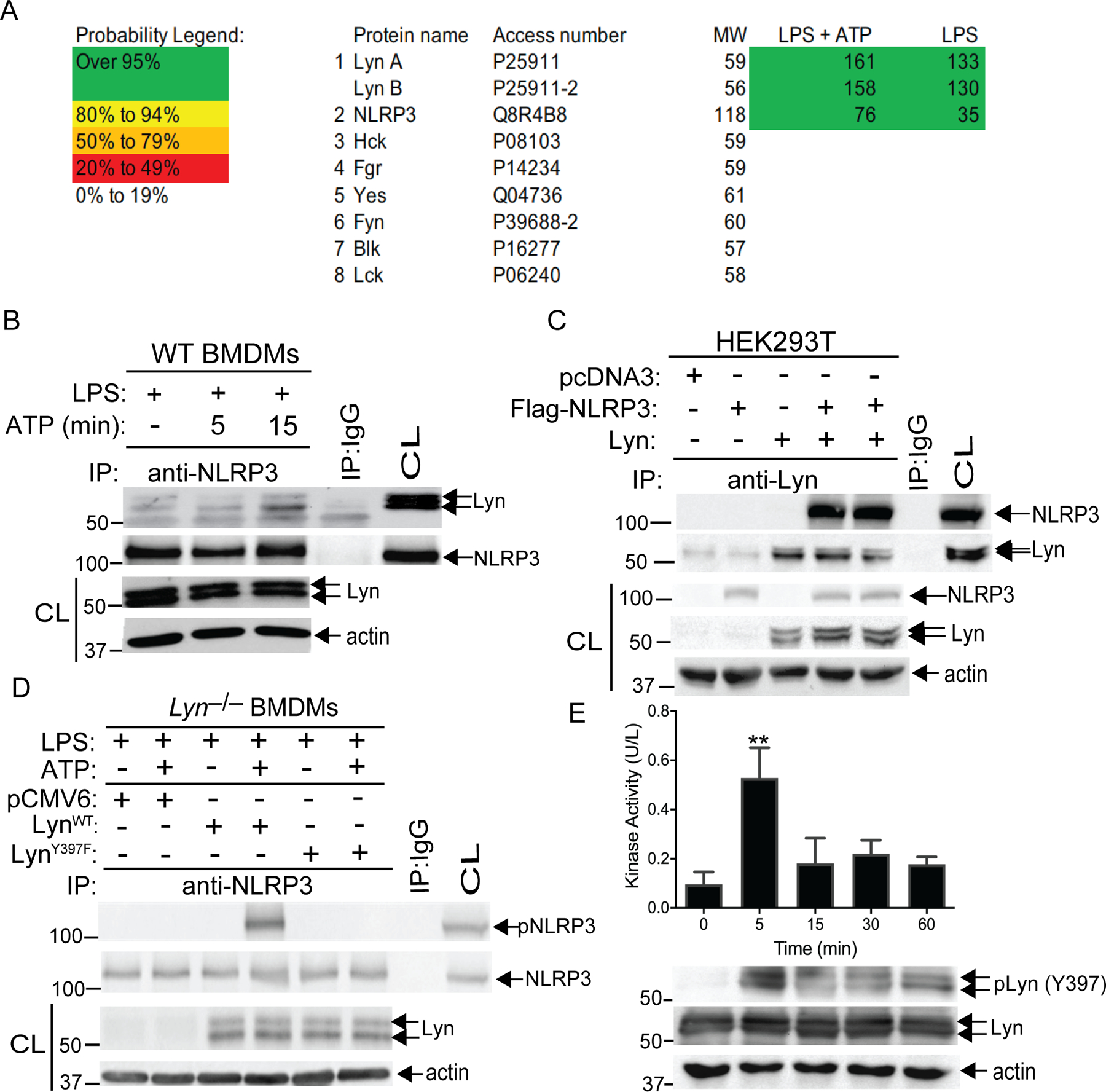
(A) LS-MS analysis of GST-NLRP3 pull-down proteins. Spectral counts of high confidence proteins are listed at the right. Thirty-four peptides with confidence >95% are assigned to two Lyn isoforms. Coverage of Lyn increased from 44% to 66% with ATP in addition to LPS. (B) Lysates from LPS-primed BMDMs treated with ATP at indicated time-points were immunoprecipitated with antibody to NLRP3 and blotted with antibodies to Lyn and NLRP3. (C) Lysates from HEK293T cells transfected with Flag-tagged NLRP3 and Lyn, were immunoprecipitated with antibody to Lyn and blotted with antibody to Flag. (D) Lysates from Lyn–/– BMDMs were transfected with the constructs expressing WT Lyn or a kinase-dead Lyn (LynY397F) upon LPS priming and ATP stimulation were immunoprecipitated with antibody to NLRP3 and blotted with antibody to pTyr. Whole cell lysates were immunoblotted with antibodies to Lyn and actin. (E) Lysates from LPS-primed BMDMs treated with ATP at indicated time-points were immunoprecipitated with antibody to Lyn, incubated with GST-NLRP3 in kinase buffer in the presence of ATP, and then detected using an EnzyChrom™ kinase assay kit. An aliquot of kinase reaction mixture was blotted with antibodies to phospho-Lyn (Tyr397) and Lyn. Data are from a representative one of two (A and D) or three (C, B and E) independent experiments. **P< 0.01, student t test
