ABSTRACT
Arthroscopic partial meniscectomy (APM) is one the most common orthopedic surgical procedures. The most common indication for APM is a degenerative meniscal tear (DMT). High-quality evidence suggests that APM does not provide meaningful benefits in patients with DMTs and may even be harmful in the longer term. This narrative review focuses on a fundamental question: considering the history and large number of these surgeries, has APM ever actually worked in patients with DMT? A truly effective treatment needs a valid disease model that would biologically and plausibly explain the perceived treatment benefits. In the case of DMT, effectiveness requires a credible framework for the pain-generating process, which should be influenced by APM. Basic research, pathoanatomy, and clinical evidence gives no support to these frameworks. Moreover, treatment of DMT with an APM does not align with the traditional practice of medicine since DMT is not a reliable diagnosis for knee pain and no evidence-based indication exists that would influence patient prognosis from APM. A plausible and robust explanation supported by both basic research and clinical evidence is that DMTs are part of an osteoarthritic disease process and do not contribute to the symptoms independently or in isolation and that symptoms are not treatable with APM. This is further supported by the fact that APM as an intervention is paradoxical because the extent of procedure and severity of disease are both inversely associated with outcome. We argue that arthroscopic treatment of DMT is largely based on a logical fallacy: post hoc ergo propter hoc.
Common surgery
Arthroscopic partial meniscectomy (APM) is one of the most common orthopedic surgical procedures (Howard 2018; Liebensteiner et al. 2020). The National Hospital Ambulatory Medical Care Survey estimated that 769,000 meniscal resections were performed in the year 2010 in the United States (Hall et al. 2017). A recent study reported an annual incidence of 291 surgeries per 100,000 persons for APM in Florida, USA (Howard 2018). In England, the annual incidence of APM was 120 per 100,000 persons in 2017 (Abram et al. 2019b). Similar incidence was reported from Finland in 2012 (Mattila et al. 2016). In recent years, the general trend in the incidence of APM has been a steady decline (Holtedahl et al. 2018, Howard 2018, Rongen et al. 2018, Lee et al. 2020, Smith et al. 2020, Karelson et al. 2021).
The most common indication for APM is a degenerative meniscal tear (DMT), which is different from acute meniscal injury. Whereas the latter is associated with acute trauma, DMTs often present after low-energy injuries or spontaneously. DMTs are thus usually considered atraumatic; the pain is incipient and the diagnosis is made after a clinical work-up related to chronic or non-acute knee pain. Due to the degenerative nature, the median age of patients treated with APM in the United States is usually between 50 and 65 and two-thirds of meniscectomies are performed in patients aged 45 or more (Hall et al. 2017, Xiao et al. 2021).
DMTs are usually located in the inner third of the posterior part of the medial meniscus and they are mainly horizontal (Pihl et al. 2017). In acute tears, a common strategy is to save the meniscus by repairing it but whether to repair or resect depends on the location of the tear. Degenerative tears, which are usually chronic in nature, do not present acutely and do not heal after repair and are thus treated by resecting the torn part of the meniscus, the supposed origin of the pain (Howell et al. 2014, Beaufils et al. 2017a, 2017b).
Evolution of APM
Knee pain or knee “derangement” following a knee injury was a clinically challenging problem in the early 1900s (Tenney 1904, Jones 1909). Meniscal (or “semilunar cartilage”) injuries, cruciate ligament tears, and patellar problems were well-established conditions at that time, but clinical examination and manual testing was the only non-invasive method to assess the possible cause of derangement. If more accurate information was needed, open knee arthrotomy was used to establish the diagnosis.
In 1889 Thomas Annandale described the very first meniscal resection to treat a meniscal tear (Annandale 1889). Removal of a torn meniscus to improve knee pain became an established procedure as early as 1900 (Jones 1909). Clinical studies at the time reported good outcomes after meniscectomy when meniscal injury was present. Several authors suggested that a torn meniscus should be removed as soon as it was identified (Wynn Parry et al. 1958, Gear 1967). They proposed that resection would mitigate the osteoarthritis progression associated with a torn meniscus. In the 1960s clinical evidence started to emerge showing that total meniscectomy led to early and accelerated degeneration and development of osteoarthritis (Gear 1967, Jackson 1968, Jackson and Abe 1972). Thereafter, more limited, i.e., partial resection was adopted to avoid the development of osteoarthritis.
Prior to arthroscopic techniques becoming available, open arthrotomy of the knee was required for meniscus excision, which is an invasive procedure associated with complications such as infection. The first attempts at endoscopic examination of the knee date back to the early twentieth century and the term arthroscopy was coined in 1912 (Jackson 1996, Macmull and Gupte 2015). It took several decades until arthroscopic techniques evolved so that simple procedures could be performed without (open) arthrotomy (Shahriaree 1992).
The first recorded arthroscopic meniscectomy of the knee was done in 1962 (Macmull and Gupte 2015). This new technique was adopted worldwide by the 1980s to treat various intra-articular knee problems. Large case series reporting favorable outcomes after arthroscopic meniscectomy started to emerge in the English language medical literature in the early 1970s (Jayson and Dixon 1968, Jackson and Abe 1972). Since late 1970s arthroscopic partial meniscectomy (APM) has remained one of the most common orthopedic procedures.
Up-to-date evidence for APM
Few comparative studies have been published that give support in favor of the APM. An early study by Merchan and Galindo (1993) concluded that arthroscopic surgery for the degenerative knee was “a useful technique” as it provided better outcome compared with nonoperative care. A major limitation of their study was that it was observational, not a randomized trial. In their RCT, Gauffin et al. (2014) reported that arthroscopic surgery provided better pain relief compared with nonoperative treatment at 1 year follow-up in people with meniscal symptoms. In a follow-up study, the trialists found that positive effects of surgery had diminished at 3- and 5-years’ follow-up and radiologic deterioration had become more common in the surgery group (Gauffin et al. 2017, Sonesson et al. 2020).
The effectiveness of APM in the treatment of DMT has been challenged by numerous rigorous randomized controlled trials (RCT). An RCT by Moseley et al., published in 2002, was the first blinded trial to assess whether arthroscopic debridement improved symptoms. The authors compared arthroscopic lavage, arthroscopic debridement (partial meniscectomy, chondroplasty, and/or loose body removal) and “incision only” (sham surgery) in osteoarthritic knees (Moseley et al. 2002). Arthroscopic surgery or lavage did not provide clinically relevant benefits compared with placebo surgery. Soon after, a trial by Kirkley et al. (2008) compared arthroscopic debridement (81% had partial meniscectomy) with physical therapy alone in patients with osteoarthrosis. Better outcome was seen in operatively treated patients in the short term but there was no difference in the longer term. At this point, it started to become clear that arthroscopic surgery and meniscectomy may not be beneficial in the long term for people with knee pain presenting with osteoarthritis. However, it was still believed that people without osteoarthritis (OA) would benefit.
Thereafter, RCTs comparing APM with usual nonoperative treatment in non-OA patients or in those with mild OA established that APM does not provide clinically important benefits (Herrlin et al. 2007, Katz et al. 2013, van de Graaf et al. 2018). The 2 largest studies included over 300 patients each and the estimates exclude any clinically important benefits. The outcomes were similar in “intention-to-treat” and “as-treated” analyses and, more importantly, the patients were not blinded to the treatment—even with the possible surgical placebo effect, APM did not provide meaningful benefits in these trials. One systematic review nevertheless concluded that there may be a small-to-moderate benefit from APM compared with physiotherapy for patients without osteoarthritis in 6–12-months’ follow-up (Abram et al. 2020).
The most rigorous evidence against benefits from APM was from the placebo-controlled FIDELITY trial published in 2013 (Sihvonen et al. 2013). There was no clinically important difference in pain or function when APM was compared with placebo surgery (lavage and inspection alone) for patients with knee pain and MRI-proven meniscus tears.
Partial meniscectomy results in objectively measurable changes in the contact pressures between joint tibiofemoral joint surfaces (Fairbank 1948, Baratz et al. 1986). Changes in knee mechanics may result in very small differences in the point estimates for patient-rated functional outcome. However, these changes do not seem to manifest as improvement in pain and function and in fact may be harmful in long-term follow-up. FIDELITY investigators reported that adjusted absolute risk difference for OA progression was 13% favoring placebo. In other words, patients having undergone APM had higher risk for OA development and progression (Sihvonen et al. 2020). A similar finding was reported by Sonesson et al. (2020) among patients randomized to APM or nonoperative treatment. Registry data from England showed that patients who had undergone an APM had a 2–3 times higher risk of total knee replacement compared with the general population (Abram et al. 2019a). This may be partly explained by confounding by indication. In a cohort study published in 2017, patients who had undergone APM were more likely to have had a knee replacement on later follow-up compared with matched patients without an APM (Rongen et al. 2017).
Considering the history and large number of surgeries done it is worth asking: has APM ever actually worked in patients with a DMT or have we been operating under an assumption based on perceived improvement post-surgery; an example of the logical fallacy—post hoc ergo propter hoc (after this, therefore because of this)?
What it takes for APM to work?
The rationale of APM ultimately is to treat knee pain. Pain is considered the hallmark symptom related to DMT but it is also a symptom of degenerative joint disease. Decreased function is usually due to exercise-related pain and discomfort. While mechanical symptoms presenting as true “locked knee” may also be considered a symptom, pain is largely driving the need for treatment. The rationale of APM is therefore to influence the pain-generating process, i.e., change the prognosis by removing the source of pain—APM is not performed to improve overall “knee health.”
Hence, is it first relevant to ask: how does the pain arise in DMT? One should consider the basic histology behind knee pain and then a disease model that would explain the role of APM from a clinical perspective. It is also relevant to consider whether APM follows the framework of clinical practice. Namely, can we start from diagnosis, which defines prognosis, and then move on to the treatment to change the prognosis? For this framework to work in patients with DMT, we need to assume that the source for pain can be identified and pain is explained by histology and a disease model.
Histology: is there biological rationale for pain?
Understanding the vascularity of the meniscus through decades of basic research has provided the rationale for treatment of meniscal injuries. The outer rim of the meniscus has good vascularity and tears in this area are usually repaired based on an assumption that as the tissue is vascularized tissue, it is therefore able to heal. In contrast, the inner third has poor or absent vascularity and tears in this location have no healing potential and hence resection is the only treatment considered.
A well-established finding is that the outer rim of the meniscus has circumferential nerve fibers (Wilson et al. 1969, Assimakopoulos et al. 1992, Mine et al. 2000, Lin et al. 2019). Some investigators have found smaller nerves running radially to the inner rim but others have reported that the inner third has no nerve endings (Day et al. 1985, Wilson et al. 1969).. The structure of innervation in the meniscus seems to be that the anterior and posterior horns are more densely innervated compared with the body of the meniscus (Lin et al. 2019). This means that DMTs are typically located in the least innervated area in the meniscus: the inner third in the posterior mid-body.
The whole human meniscus has a role in knee nociception and especially in OA. Nerve endings present in the meniscus are substance P positive (Mine et al. 2000, Lin et al. 2019). Substance P has an important role in pain perception and nociception. Substance P positive fibers are present in both perimeniscal nerves and in those running radially in the intermediate zone. Recent evidence suggests that nerve fibers positive for calcitonin-gene related peptide (CGRP) grow into the outer rim of the meniscus and also cartilage during OA (Hunter et al. 2009, Ashraf et al. 2011). This process has been proposed as one possible explanation for pain in OA as CGRP is an important transmitter in nociception.
Disease framework: can we explain pain?
The foundation of modern medicine and clinical practice is a disease model (Tinetti and Fried 2004, Agusti 2018). A disease model is needed to connect signs and symptoms to a diagnosis, which guides the treatment of the disease. In other words, we need a theoretical framework that plausibly connects our diagnosis to the prognosis and explains the causal pathway of the treatment effect.
Regarding DMT, 2 different theories can be proposed (Figure 1). The first states that DMT is an isolated process or condition responsible for the pain. This means that DMT is a disease, a meniscopathy. This framework suggests that pain in meniscopathy is caused by a meniscal tear. Under this framework, the pain is resolved with APM. This framework would be supported by empirical evidence if APM was better than placebo surgery or no surgery. As suggested above, this is not supported by the evidence.
Figure 1.
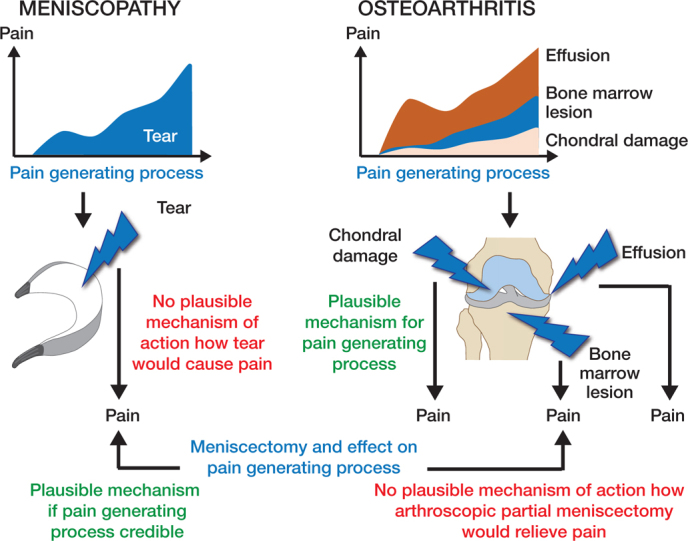
2 possible disease models related to DMT.
Grönblad et al. (1985) suggested that meniscectomy could work through denervation. This theory is, however, weak and clinical studies do not support this mechanism of action. Because meniscectomy should be as conservative as possible, the meniscus preserves most of its innervation and is not totally denervated. If DMT was an isolated process, how could resection of a non-innervated and avascular tissue affect pain? More importantly, no clinical study supports the proposition that occurrence of DMT would result in knee pain or that DMT is a defined independent clinical entity. In fact, clinical studies show that development of knee pain is not associated with the development of DMT and that “meniscal symptoms” are merely a correlate for cartilage damage (Bhattacharyya et al. 2003, Englund et al. 2007, Hare et al. 2017, MacFarlane et al. 2021).
The second, an opposing framework, proposes that DMT is not an isolated disease (meniscopathy) but belongs to a spectrum of the osteoarthritic process. Under this framework, similar to removal of an osteophyte, APM has no independent role because it is not intervening or mediating the pain-generating process. Empirical evidence supports this proposition and would explain why APM does not relieve pain. Pain in OA is explained by a variety of different entities that interact as a continuum.
Bone marrow lesions are well described radiological findings adjacent to degenerative joint diseases and strong evidence from longitudinal studies show that progressive bone marrow lesion development is associated with development of knee pain (Felson et al. 2007). Synovitis is also a plausible cause for pain since knee synovium, especially in non-arthritic knees, is highly innervated (Mapp 1995). Synovitis has been associated with progressive OA, development of symptoms, and chemokine expression related to nociceptive stimuli in multiple longitudinal studies (Ayral et al. 2005, Scanzello et al. 2011). On a molecular level, degradation of cartilage results in release of damage-associated molecular pattern molecules and alarmins, which in turn is associated with the release of proinflammatory cytokines, such as tumor necrosis factor and interleukins (Liu-Bryan and Terkeltaub 2015, Eitner et al. 2017). These mediators, as with other proinflammatory mediators, have the potential to reduce the excitation threshold in high-threshold nociceptive neurons, thus making them more likely to respond to noxious and non-noxious stimuli explaining the most plausible pain-generating process (Miller et al. 2015, Fu et al. 2018). From a disease model perspective, the most plausible model and theory is that DMT is an early part of the degenerative process in OA in which APM plays no role.
Practice of medicine and diagnosis
Practice of medicine traditionally builds on 3 concepts: (1) diagnosis, (2) prognosis, and (3) treatment (Chauffard 1913, Tinetti and Fried 2004, Croft et al. 2015) (Figure 2). Diagnosis is the fundamental concept dividing people into sick and healthy; those with a disease and those without. Further steps, prognosis, and treatment in this framework follow the diagnosis.
Figure 2.
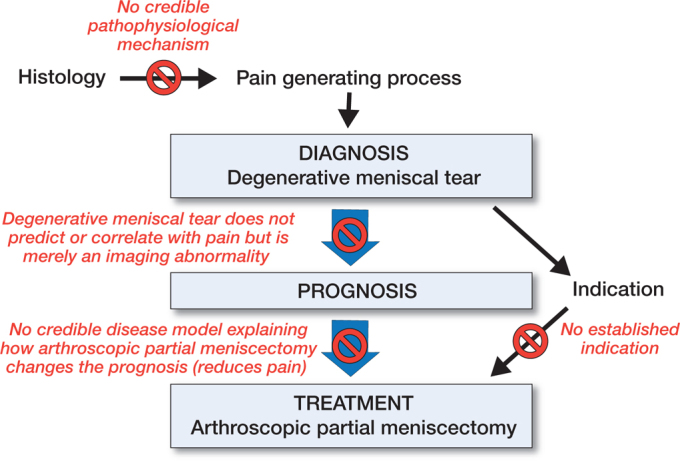
Traditional view in practice of medicine and APM as a case example.
Before we consider any treatment, we need a diagnosis. Clinical work-up and diagnostics in patients with musculoskeletal pain are based on history, clinical examination, and use of imaging modalities. People seek help not because they have DMT, but because they have knee pain. If APM worked, it would mean that DMT is a reliable diagnosis and a disease, meaning that DMT explains the knee pain and is not just an incidental imaging finding. As outlined above, no valid clinical evidence supports the proposition that DMT is an actual clinical disease entity.
DMT is a poor indicator for knee pain at a population level. Englund et al. (2008) estimated that meniscal tears are seen in one-third of males and one-sixth of females aged 50–59 years, with prevalence increasing with age. Horga et al. (2020) found tears in one-third of 230 asymptomatic people with an average age of 44 years. Tears are slightly more common in patients with knee symptoms compared with asymptomatic patients in people without radiological osteoarthritis. However, in people with radiological osteoarthritis, tears were equally common regardless of symptoms (Englund et al. 2008).
Considering the commonness of APM, the validity of diagnostic imaging and clinical findings in DMT has been investigated surprisingly rarely. Campbell et al. (2014) investigated whether the location of pain correlates with intra-articular findings in patients diagnosed with DMT, and found no correlation; only 15 of 50 patients reporting medial-sided pain had arthroscopically confirmed isolated medial compartment pathology. More recently Farina et al. (2021) reported that meniscal and mechanical symptoms were predictors of cartilage damage and not meniscal pathology.
Joint-line tenderness has been traditionally considered as a hallmark sign of DMT, as was also recently concluded in a consensus statement by Lyman et al. (2012). Again, evidence does not uniformly support this. Numerous studies have investigated the clinical accuracy of McMurray’s test, jointline tenderness. and Thessaly’s test (Smoak et al. 2020). The results have varied and on average the accuracy of these tests is poor. A major shortcoming in these studies has been that they have not discriminated between degenerative and clearly traumatic meniscal tears and DMTs (Smith et al. 2015). The average age of patients in these studies has been well below 40 years. Patient inclusion has been also poorly described.
Most recently, Décary et al. (2018) looked at patients with traumatic or degenerative meniscal tear. Medial joint-line tenderness increased the likelihood of traumatic tear but not DMT. Moreover, a combination of medial-sided pain, progressive onset of pain, pain while pivoting, normal alignment, and full extension had moderate ability to discriminate people with DMT from traumatic tears. Importantly, this combination of symptoms describes mild osteoarthrosis and is not specific to meniscal tears.
To conclude, we cannot identify and diagnose DMT by the presence of symptoms or clinical findings and DMT seen on MRI does not correlate reliably with symptoms.
From diagnosis of DMT to an indication for APM
Does the diagnosis of DMT tell us anything about the prognosis? If a surgical intervention improves the prognosis with a certain diagnosis, then the diagnosis can potentially be considered an “indication for surgery.” Surgical indication, in other words, is a factor supposedly interacting with the association between the diagnosis and prognosis, i.e., intervention modifies the outcome (compared with no intervention). The intervention should not be performed if the prognosis remains unchanged or becomes worse compared with no intervention. This is the fundamental principle in “science of prognosis” (Croft et al. 2015). which has been proposed to replace the concept of “science of diagnosis.” In the science of prognosis, the diagnosis is a fundamental way to convey prognostic information.
Before trials refuted the benefits of APM in osteoarthritis, APM was a routine treatment in patients with degenerative symptomatic joint disease. Thereafter, the indication has mainly been “non-OA” patients with DMT, patients we postulate as having so-called “isolated” meniscal tears (Beaufils et al. 2017a, Stone et al. 2017a). As several RCTs suggest that the prognosis of DMT is not altered with surgery, meniscal abnormality on MRI or arthroscopy is not an indication for APM from a prognosis perspective—it does not improve the prognosis, and may be even be harmful in the long term.
Several guidelines for DMTs and APMs have been published (Table). They outline a spectrum of indications for APM. Most guidelines have concluded that nonoperative treatment should be the primary choice in DMTs. The current paradigm is to use APM to treat those patients who do not improve with non-operative treatment. Accordingly, “failed nonoperative treatment” is considered as an indication for surgery. Interestingly, this was considered some 135 years ago when T. Annandale wrote that “[operation for displaced semilunar cartilage] may now become an established means of treatment when the more simple methods fail” (Annandale 1889). No evidence to date supports the proposition that APM would alter the prognosis specifically in patients who have not responded to 3 months of nonoperative treatment as suggested by the guidelines.
Summary of diagnostics related to DMT and indications for APM
An Expert Consensus Statement (Hohmann et al. 2020)
British Association for Surgery of the Knee (BASK) Meniscal Consensus Project (Abram et al. 2019a)
ESSKA Meniscus Consensus Project (Kopf et al. 2020)
Australian Knee Society Position Statement (Australian Knee Society 2017)
ISAKOS: Consensus statements across three continents (Stone et al. 2017a)
Dutch Orthopaedic Association (Van Arkel et al. 2021)
|
“Mechanical symptoms” together with DMT have been considered an indication for APM. A consensus statement from 2012 did not consider mechanical symptoms worthy of discussion because “there would be nearly uniform agreement that patients with such symptoms would require surgery” (Lyman et al. 2012). No study prior to that statement had tested this hypothesis; the benefits were assumed based on anecdotal clinical experience, biomechanical reasoning, and an old paradigm of medicine stipulating that removing the pathophysiology cures the disease (Evidence-Based Medicine Working Group 1992). Recent evidence does not support this consensus. Thorlund et al. (2019) reported arthroscopic findings among over 800 patients and could not detect a difference in prevalence of patient-reported mechanical symptoms in patients with or without a meniscal tear. In other words, they could not associate a disease called “DMT with mechanical symptoms” with a pathological process. Sihvonen et al. (2018) reported in the follow-up study of the FIDELITY trial that a mechanical symptom was not an effect modifier in APM. Until further evidence emerges, it is a valid assumption that mechanical symptoms are not an unequivocal indication for APM.
Treatment should influence prognosis and diagnosis precedes prognosis. The meaning of the diagnosis should entail both pathology (source of symptoms) and a biologically plausible mechanism to explain the treatment effect. None of this is valid for DMT and APM. DMT as a disease does not align with fundamental aspects in clinical medicine: it does not affect the prognosis, and removal of the pathology does not affect the symptoms. DMT is simply an imaging finding in OA; we cannot separate “healthy” from “sick” unless the imaging abnormality is labeled as disease.
Paradoxical effect of APM
The dose–response relationship is a natural phenomenon in medicine. Usually, the more severe the disease, the more relief or improvement is achieved by removing the pathology. A well-established finding regarding total knee replacement is that dissatisfaction is more likely if the OA is mild (Stone et al. 2017b, van de Water et al. 2019). APM entails paradoxical effects in this respect (Figure 3). Total meniscectomy leads to more rapid degeneration in the knee joint compared with partial resection and this was also the rationale for adaption of partial meniscectomy (Northmore-Ball et al. 1983, Englund and Lohmander 2004, Kise et al. 2019). Even in subtotal meniscectomy, the extent of excision correlates with the outcome: the larger the excision, the poorer the outcome (Rockborn and Gillquist 1995, Englund and Lohmander 2004, Kise et al. 2019). In this analogy, it would seem reasonable to excise nothing, assuming that it would lead to a better outcome. Obviously, this is irrational if removal of pathological tissue is the purpose of the treatment. The extent of the excision is confounded by the underlying disease: the larger the damage, the larger the excision needed. This means that extrusion of the meniscus and larger tears predict less benefit, not more benefit, as should be expected assuming a dose–response relationship (Kise et al. 2019). Again, the theoretical framework of meniscopathy is not supported as the severity of the disease actually inversely predicts the outcome, although the cause for pain is supposedly removed.
Figure 3.
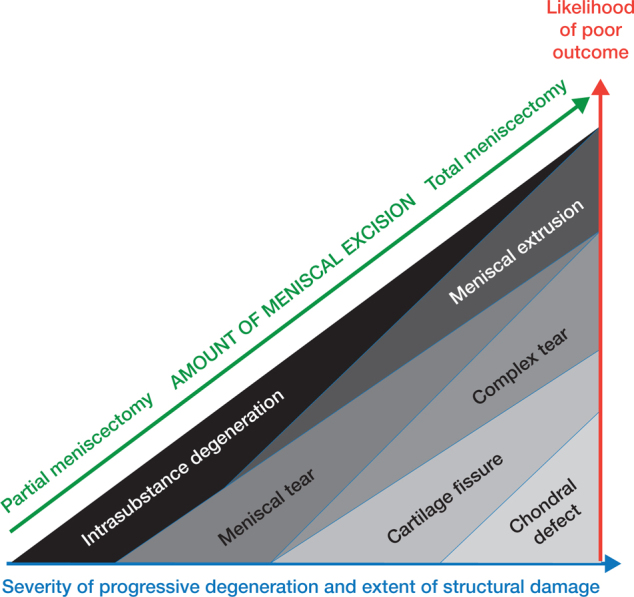
Paradoxical effect of APM. Severity of the degeneration correlates with poor outcome (red and blue arrows). At the same time the larger the excision, the poorer the outcome despite the disease being more severe (green arrow). APM as an intervention is paradoxical since the extent of procedure and severity of disease are both inversely associated with outcome, meaning that as little as possible should be excised but then the underlying pathology is not addressed.
Contradicting the clinical experience
The FIDELITY trial caused a pushback from the orthopedic community defending APM (Krogsgaard et al. 2014, Lubowitz et al. 2014, Rossi et al. 2014, Sutherland et al. 2014, Sochacki et al. 2020). Justification for dismissing experimental evidence that did not favor APM was subjective experience (observational evidence): in people who seek help due to knee pain, DMT is found and clinical status improves after the APM (post hoc ergo propter hoc).
Further criticism claims that RCTs must be flawed as they contradict observations: the population randomized was not generalizable or the studies were biased towards null through such things as genetics, crossovers, or co-interventions (Krogsgaard et al. 2014, Lubowitz et al. 2014, Rossi et al. 2014, Sutherland et al. 2014, Sochacki et al. 2020). The authors who suggest that the evidence is biased seldom acknowledge the true estimate for efficacy. Put another way, they leave unclear what the true effect is from which the treatment effect estimates are biased. However, the estimates from rigorous trials seem to be consistent in that the lack of efficacy is a reproducible finding (Abram et al. 2020). It is also surprising that the supposed true efficacy remains hidden despite the extent of research efforts.
Several causes may explain the clinical observations (Figure 4). First, people likely seek help when they are at a “peak” in the fluctuating symptom curve. Whatever is done, they are likely to benefit due to regression to mean. Regression to mean is a recognized phenomenon where extreme values, either low or high, usually regress towards the mean (become less extreme) when measured for a second time (Barnett et al. 2005). This fits well in degenerative conditions where clinical status is often fluctuating.
Figure 4.
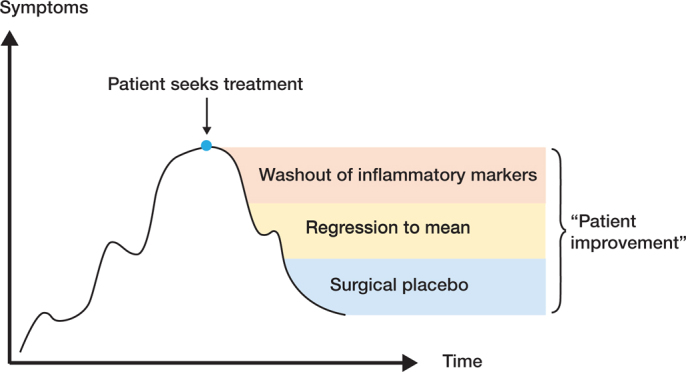
Why do patients improve after APM? Figure 4. Why do patients improve after APM?
Second, part of the transient improvement seen in patients having undergone an APM may be due to washout of inflammatory mediators. As said, OA is a disease that affects the whole joint. Currently OA is believed to be an inflammatory disease that is mediated by inflammatory markers measurable in the synovial fluid. These inflammatory markers are also responsible for pain sensation, which is transmitted through nerve endings in the synovia. During APM the knee joint is lavaged and this results in washout of intra-articular inflammatory mediators present in the joint surfaces and synovial fluid. Moreover, if APM truly removes the pain-generating process, why are good outcomes not seen in the first few weeks? The treatment response, namely reduction in pain and improvement in knee function, takes several months to manifest.
Lastly, one possible source of perceived benefit is other non-specific effects of surgery (contextual and placebo effects) (Harris 2021). In surgery, placebo effect is suggested to be large (Doherty and Dieppe 2009). However, the evidence is limited as the measurement of contextual and placebo effects is difficult (Hróbjartsson and Gøtzsche 2001).
Final remarks
Basic research of pathoanatomy does not support the theory that DMT is an isolated disease that can be adequately treated with APM. Clinical evidence does not support a disease framework named meniscopathy. Furthermore, APM as an intervention is paradoxical because the extent of procedure and severity of disease are both inversely associated with outcome. A plausible and robust explanation supported by both basic research and clinical evidence is that DMTs are part of OA and do not contribute to the symptoms independently or in isolation and therefore the symptoms are not treatable with APM. If DMT caused pain, removal would yield benefits that are detectable in clinical trials, but this has not been the case. Until a subpopulation that benefits from APM is identified, it has no role in the treatment of degenerative meniscal tears.
Competing interests
None of the authors have any relevant conflicts of interest.
Acknowledgments
AR conceived the presented idea and wrote the initial drafted. TK and IH gave critical comments on the draft and edited the draft. All authors discussed the manuscript and contributed to the final manuscript.
Acta thanks Paul W Ackermann and Freddie Fu for help with peer review of this study.
References
- Abram S G F, Judge A, Beard D J, Carr A J, Price A J. Long-term rates of knee arthroplasty in a cohort of 834 393 patients with a history of arthroscopic partial meniscectomy. Bone Joint J 2019a; 101-B(9): 1071–80. [DOI] [PubMed] [Google Scholar]
- Abram S G F, Judge A, Beard D J, Wilson H A, Price A J. Temporal trends and regional variation in the rate of arthroscopic knee surgery in England: analysis of over 1.7 million procedures between 1997 and 2017. Has practice changed in response to new evidence? Br J Sports Med 2019b; 53(24): 1533–8. [DOI] [PubMed] [Google Scholar]
- Abram S G F, Hopewell S, Monk A P, Bayliss L E, Beard D J, Price A J. Arthroscopic partial meniscectomy for meniscal tears of the knee: a systematic review and meta-analysis. Br J Sports Med 2020; 54(11): 652–63. [DOI] [PubMed] [Google Scholar]
- Agusti A. The disease model: implications for clinical practice. Eur Respir J 2018; 51(4): 1800188. [DOI] [PubMed] [Google Scholar]
- Annandale T. Excision of the internal semilunar cartilage, resulting in perfect restoration of the joint-movements. BMJ 1889; 1(1467): 291–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf S, Wibberley H, Mapp P I, Hill R, Wilson D, Walsh D A. Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Ann Rheum Dis 2011; 70(3): 523–9. [DOI] [PubMed] [Google Scholar]
- Assimakopoulos A P, Katonis P G, Agapitos M V, Exarchou E I. The innervation of the human meniscus. Clin Orthop Relat Res 1992; (275): 232–6. [PubMed] [Google Scholar]
- Australian Knee Society . Position statement from the australian knee society on arthroscopic surgery of the knee, including reference to the presence of osteoarthritis or degenerative joint disease: updated october 2016. Orthop J Sports Med 2017; 5(9): 2325967117728677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayral X, Pickering E H, Woodworth T G, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr Cartil 2005; 13(5): 361–7. [DOI] [PubMed] [Google Scholar]
- Baratz M E, Fu F H, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med 1986; 14(4): 270–5. [DOI] [PubMed] [Google Scholar]
- Barnett A G, van der Pols J C, Dobson A J. Regression to the mean: what it is and how to deal with it. Int J Epidemiol 2005; 34(1): 215–20. [DOI] [PubMed] [Google Scholar]
- Beaufils P, Becker R, Kopf S, Englund M, Verdonk R, Ollivier M, et al. Surgical management of degenerative meniscus lesions: the 2016 ESSKA meniscus consensus. Knee Surg Sports Traumatol Arthrosc 2017a; 25(2): 335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufils P, Becker R, Kopf S, Matthieu O, Pujol N. The knee meniscus: management of traumatic tears and degenerative lesions. EFORT Open Reviews 2017b; 2(5): 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya T, Gale D, Dewire P, Totterman S, Gale M E, McLaughlin S, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am 2003; 85(1): 4–9. [DOI] [PubMed] [Google Scholar]
- Campbell J, Harte A, Kerr D P, Murray P. The location of knee pain and pathology in patients with a presumed meniscus tear: preoperative symptoms compared to arthroscopic findings. Ir J Med Sci 2014; 183(1): 23–31. [DOI] [PubMed] [Google Scholar]
- Chauffard A. Address in medicine, ON MEDICAL PROGNOSIS: ITS METHODS, ITS EVOLUTION, ITS LIMITATIONS: delivered at the seventeenth international congress of medicine. BMJ 1913; 2(2745): 286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft P, Altman D G, Deeks J J, Dunn KM, Hay A D, Hemingway H, et al. The science of clinical practice: disease diagnosis or patient prognosis? Evidence about “what is likely to happen” should shape clinical practice. BMC Med 2015; 13: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B, Mackenzie WG, Shim SS, Leung G. The vascular and nerve supply of the human meniscus. Arthroscopy 1985; 1(1): 58–62. [DOI] [PubMed] [Google Scholar]
- Décary S, Fallaha M, Frémont P, Martel-Pelletier J, Pelletier J-P, Feldman D E, et al. Diagnostic validity of combining history elements and physical examination tests for traumatic and degenerative symptomatic meniscal tears. PM R 2018; 10(5): 472–82. [DOI] [PubMed] [Google Scholar]
- Doherty M, Dieppe P. The “placebo” response in osteoarthritis and its implications for clinical practice. Osteoarthritis Cartilage 2009; 17(10): 1255–62. [DOI] [PubMed] [Google Scholar]
- Eitner A, Hofmann G O, Schaible H-G. Mechanisms of osteoarthritic pain: studies in humans and experimental models. Front Mol Neurosci 2017; 10: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund M, Lohmander L S. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum 2004. ep; 50(9): 2811–19. [DOI] [PubMed] [Google Scholar]
- Englund M, Niu J, Guermazi A, Roemer F W, Hunter D J, Lynch J A, et al. Effect of meniscal damage on the development of frequent knee pain, aching, or stiffness. Arthritis Rheum 2007; 56(12): 4048–54. [DOI] [PubMed] [Google Scholar]
- Englund M, Guermazi A, Gale D, Hunter D J, Aliabadi P, Clancy M, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med 2008; 359(11): 1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evidence-Based Medicine Working Group . Evidence-based medicine: a new approach to teaching the practice of medicine. JAMA 1992; 268(17): 2420–5. [DOI] [PubMed] [Google Scholar]
- Fairbank T J. Knee joint changes after meniscectomy. J Bone Joint Surg Br 1948; 30B(4): 664–70. [PubMed] [Google Scholar]
- Farina E M, Lowenstein N A, Chang Y, Arant K R, Katz J N, Matzkin E G. Meniscal and mechanical symptoms are associated with cartilage damage, not meniscal pathology. J Bone Joint Surg Am 2021; 103(5): 381–8. [DOI] [PubMed] [Google Scholar]
- Felson D T, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum 2007; 56(9): 2986–92. [DOI] [PubMed] [Google Scholar]
- Fu K, Robbins S R, McDougall J J. Osteoarthritis: the genesis of pain. Rheumatology 2018; 57(Suppl_4): iv43–iv50. [DOI] [PubMed] [Google Scholar]
- Gauffin H, Tagesson S, Meunier A, Magnusson H, Kvist J. Knee arthroscopic surgery is beneficial to middle-aged patients with meniscal symptoms: a prospective, randomised, single-blinded study. Osteoarthritis Cartilage 2014; 22(11): 1808–16. [DOI] [PubMed] [Google Scholar]
- Gauffin H, Sonesson S, Meunier A, Magnusson H, Kvist J. Knee arthroscopic surgery in middle-aged patients with meniscal symptoms: a 3-year follow-up of a prospective, randomized study. Am J Sports Med 2017; 45(9): 2077–84. [DOI] [PubMed] [Google Scholar]
- Gear M W. The late results of meniscectomy. Br J Surg 1967; 54(4): 270–2. [DOI] [PubMed] [Google Scholar]
- Grönblad M, Korkala O, Liesi P, Karaharju E. Innervation of synovial membrane and meniscus. Acta Orthop Scand 1985; 56(6): 484–6. [DOI] [PubMed] [Google Scholar]
- Hall M J, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: united states, 2010. Natl Health Stat Report 2017; (102): 1–15. [PubMed] [Google Scholar]
- Hare K B, Stefan Lohmander L, Kise N J, Risberg M A, Roos E M. >Middleaged patients with an MRI-verified medial meniscal tear report symptoms commonly associated with knee osteoarthritis. Acta Orthop 2017; 88(6): 664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris I. When the placebo effect is not an effect. Acta Orthop 2021; 92 (5): 501–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlin S, Hållander M, Wange P, Weidenhielm L, Werner S. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg Sports Traumatol Arthrosc 2007; 15(4): 393–401. [DOI] [PubMed] [Google Scholar]
- Hohmann E, Angelo R, Arciero R, Bach B R, Cole B, Cote M, et al. Degenerative meniscus lesions: an expert consensus statement using the modified delphi technique. Arthroscopy 2020; 36(2): 501–12. [DOI] [PubMed] [Google Scholar]
- Holtedahl R, Brox J I, Aune A K, Nguyen D, Risberg M A, Tjomsland O. Changes in the rate of publicly financed knee arthroscopies: an analysis of data from the Norwegian patient registry from 2012 to 2016. BMJ Open 2018; 8(6): e021199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horga L M, Hirschmann A C, Henckel J, Fotiadou A, Di Laura A, Torlasco C, et al. Prevalence of abnormal findings in 230 knees of asymptomatic adults using 3.0 T MRI. Skeletal Radiol 2020; 49(7): 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D H. Trends in the use of knee arthroscopy in adults. JAMA Intern Med 2018; 178(11): 1557–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell R, Kumar N S, Patel N, Tom J. Degenerative meniscus: pathogenesis, diagnosis, and treatment options. World J Orthop 2014; 5(5): 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hróbjartsson A, Gøtzsche P C. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med 2001; 344(21): 1594–1602. [DOI] [PubMed] [Google Scholar]
- Hunter D J, McDougall J J, Keefe F J. The symptoms of osteoarthritis and the genesis of pain. Med Clin North Am 2009; 93(1): 83–100, xi. [DOI] [PubMed] [Google Scholar]
- Jackson J P. Degenerative changes in the knee after meniscectomy. BMJ 1968; 2(5604): 525–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R W. From the scalpel to the scope: the history of arthroscopy. Baylor University Medical Center Proceedings 1996; 9(4): 77–9. [Google Scholar]
- Jackson R W, Abe I. The role of arthroscopy in the management of disorders of the knee: an analysis of 200 consecutive examinations. J Bone Joint Surg Br 1972; 54(2): 310–22. [PubMed] [Google Scholar]
- Jayson M I, Dixon A S. Arthroscopy of the knee in rheumatic diseases. Ann Rheum Dis 1968; 27(6): 503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. II. Derangements of the knee: based upon a personal experience of over five hundred operations. Ann Surg 1909; 50(6): 969–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelson M C, Jokihaara J, Launonen A P, Huttunen T, Mattila V M. Lower nationwide rates of arthroscopic procedures in 2016 compared with 1997 (634925 total arthroscopic procedures): has the tide turned? Br J Sports Med 2021; 55(18): 1018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J N, Brophy R H, Chaisson C E, de Chaves L, Cole B J, Dahm D L, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med 2013; 368(18): 1675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkley A, Birmingham T B, Litchfield R B, Giffin J R, Willits K R, Wong C J, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2008; 359(11): 1097–1107. [DOI] [PubMed] [Google Scholar]
- Kise N J, Aga C, Engebretsen L, Roos E M, Tariq R, Risberg M A. Complex tears, extrusion, and larger excision are prognostic factors for worse outcomes 1 and 2 years after arthroscopic partial meniscectomy for degenerative meniscal tears: a secondary explorative study of the surgically treated group from the Odense-Oslo Meniscectomy Versus Exercise (OMEX) Trial. Am J Sports Med 2019; 47(10): 2402–11. [DOI] [PubMed] [Google Scholar]
- Kopf S, Beaufils P, Hirschmann MT, Rotigliano N, Ollivier M, Pereira H, et al. Management of traumatic meniscus tears: the 2019 ESSKA meniscus consensus. Knee Surg Sports Traumatol Arthrosc 2020; 28(4): 1177–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsgaard M R, Lind M, Jørgensen U. A positive viewpoint regarding arthroscopy for degenerative knee conditions. Acta Orthop 2014; 85(6): 681–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S M S, Awal W, Vertullo C. Changing practice: incidence of non-reconstructive arthroscopic knee surgery in people over 50 years of age, Australia, 2008–2018. Med J Aust 2020; 212(1): 29–30. [DOI] [PubMed] [Google Scholar]
- Liebensteiner M C, Khosravi I, Hirschmann M T, Heuberer P R, Board of the AGA-Society of Arthroscopy and Joint-Surgery, Thaler M. Massive cutback in orthopaedic healthcare services due to the COVID-19 pandemic. Knee Surg Sports Traumatol Arthrosc 2020; 28(6): 1705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zhang K, Li Q, Li J, Xu B. Innervation of nociceptors in intact human menisci along the longitudinal axis: semi-quantitative histological evaluation and clinical implications. BMC Musculoskelet Disord 2019; 20(1): 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol 2015; 11(1): 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubowitz J H, Provencher M T, Rossi M J. Could the New England Journal of Medicine be biased against arthroscopic knee surgery? Part 2. Arthroscopy 2014; 30(6): 654–5. [DOI] [PubMed] [Google Scholar]
- Lyman S, Oh L S, Reinhardt K R, Mandl L A, Katz J N, Levy B A, et al. Surgical decision making for arthroscopic partial meniscectomy in patients aged over 40 years. Arthroscopy 2012; 28(4): 492–501.e1. [DOI] [PubMed] [Google Scholar]
- MacFarlane L A, Yang H, Collins J E, Brophy R H, Cole B J, Spindler K P, et al. Association between baseline “meniscal symptoms” and outcomes of operative and non-operative treatment of meniscal tear in patients with osteoarthritis. Arthritis Care Res (Hoboken) 2021. Mar 1. doi: 10.1002/acr.24588. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmull S, Gupte C M. (ii) Basic knee arthroscopy: a brief history, surgical techniques and potential complications. Orthop Trauma 2015; 29(1): 6–11. [Google Scholar]
- Mapp P I. Innervation of the synovium. Ann Rheum Dis 1995; 54(5): 398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila V M, Sihvonen R, Paloneva J, Felländer-Tsai L. Changes in rates of arthroscopy due to degenerative knee disease and traumatic meniscal tears in Finland and Sweden. Acta Orthop 2016; 87(1): 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchan E C R, Galindo E. Arthroscope-guided surgery versus nonoperative treatment for limited degenerative osteoarthritis of the femorotibial joint in patients over 50 years of age: a prospective comparative study. Arthroscopy 1993; 9(6): 663–7. [DOI] [PubMed] [Google Scholar]
- Miller R E, Tran P B, Obeidat A M, Raghu P, Ishihara S, Miller R J, et al. The role of peripheral nociceptive neurons in the pathophysiology of osteoarthritis pain. Curr Osteoporos Rep 2015; 13(5): 318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine T, Kimura M, Sakka A, Kawai S. Innervation of nociceptors in the menisci of the knee joint: an immunohistochemical study. Arch Orthop Trauma Surg 2000; 120(3–4): 201–4. [DOI] [PubMed] [Google Scholar]
- Moseley J B, O’Malley K, Petersen N J, Menke T J, Brody B A, Kuykendall D H, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002; 347(2): 81–8. [DOI] [PubMed] [Google Scholar]
- Northmore-Ball M D, Dandy D J, Jackson R W. Arthroscopic, open partial, and total meniscectomy: a comparative study. J Bone Joint Surg Br 1983; 65(4): 400–4. [DOI] [PubMed] [Google Scholar]
- Pihl K, Englund M, Lohmander L S, Jørgensen U, Nissen N, Schjerning J, et al. Signs of knee osteoarthritis common in 620 patients undergoing arthroscopic surgery for meniscal tear. Acta Orthop 2017; 88(1): 90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockborn P, Gillquist J. Outcome of arthroscopic meniscectomy: a a 13-year physical and radiographic follow-up of 43 patients under 23 years of age. Acta Orthop Scand 1995; 66(2): 113–17. [DOI] [PubMed] [Google Scholar]
- Rongen J J, Rovers M M, van Tienen T G, Buma P, Hannink G. Increased risk for knee replacement surgery after arthroscopic surgery for degenerative meniscal tears: a multi-center longitudinal observational study using data from the osteoarthritis initiative. Osteoarthritis Cartilage 2017; 25(1): 23–9. [DOI] [PubMed] [Google Scholar]
- Rongen J J, van Tienen T G, Buma P, Hannink G. Meniscus surgery is still widely performed in the treatment of degenerative meniscus tears in The Netherlands. Knee Surg Sports Traumatol Arthrosc 2018; 26(4): 1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M J, D’Agostino R B, Provencher M T, Lubowitz J H. Could the New England Journal of Medicine be biased against arthroscopic knee surgery? Arthroscopy 2014; 30(5): 536–7. [DOI] [PubMed] [Google Scholar]
- Scanzello C R, McKeon B, Swaim B H, DiCarlo E, Asomugha E U, Kanda V, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum 2011; 63(2): 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahriaree H, ed. O’Connor’s textbook of arthroscopic surgery, 2nd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 1992. [Google Scholar]
- Sihvonen R, Paavola M, Malmivaara A, Itälä A, Joukainen A, Nurmi H, et al. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med 2013; 369(26): 2515–24. [DOI] [PubMed] [Google Scholar]
- Sihvonen R, Paavola M, Malmivaara A, Itälä A, Joukainen A, Nurmi H, et al. Arthroscopic partial meniscectomy versus placebo surgery for a degenerative meniscus tear: a 2-year follow-up of the randomised controlled trial. Ann Rheum Dis 2018; 77(2): 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvonen R, Paavola M, Malmivaara A, Itälä A, Joukainen A, Kalske J, et al. Arthroscopic partial meniscectomy for a degenerative meniscus tear: a 5 year follow-up of the placebo-surgery controlled FIDELITY (Finnish Degenerative Meniscus Lesion Study) trial. Br J Sports Med 2020; 54(22): 1332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B E, Thacker D, Crewesmith A, Hall M. Special tests for assessing meniscal tears within the knee: a systematic review and meta-analysis. Evid Based Med 2015; 20(3): 88–97. [DOI] [PubMed] [Google Scholar]
- Smith L, Barratt A, Buchbinder R, Harris I A, Doust J, Bell K. Trends in knee magnetic resonance imaging, arthroscopies and joint replacements in older Australians: still too much low-value care? ANZ J Surg 2020; 90(5): 833–9. [DOI] [PubMed] [Google Scholar]
- Smoak J B, Matthews J R, Vinod A V, Kluczynski M A, Bisson L J. An up-to-date review of the meniscus literature: a systematic summary of systematic reviews and meta-analyses. Orthop J Sports Med 2020; 8(9): 2325967120950306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochacki K R, Mather R C, Nwachukwu B U, Dong D, Nho S J, Cote MP, et al. Sham surgery studies in orthopedic surgery may just be a sham: a systematic review of randomized placebo-controlled trials. Arthroscopy 2020. Epub May 14. [DOI] [PubMed] [Google Scholar]
- Sonesson S, Kvist J, Yakob J, Hedevik H, Gauffin H. Knee arthroscopic surgery in middle-aged patients with meniscal symptoms: a 5-year follow-up of a prospective, randomized study. Orthop J Sports Med 2020; 8(1): 2325967119893920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J A, Salzler M J, Parker D A, Becker R, Harner C D. Degenerative meniscus tears—assimilation of evidence and consensus statements across three continents: state of the art. J ISAKOS 2017a; 2(2): 108–19. [Google Scholar]
- Stone O D, Duckworth A D, Curran D P, Ballantyne J A, Brenkel I J. Severe arthritis predicts greater improvements in function following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2017b; 25(8): 2573–9. [DOI] [PubMed] [Google Scholar]
- Sutherland A G, Cuthbertson B H, Campbell M. Sham surgery studies. Arthroscopy 2014; 30(11): 1389. [DOI] [PubMed] [Google Scholar]
- Tenney B. I. The anatomy and surgery of the internal derangements of the knee-joint. Based on a study of 150 dissected joints and the literature. Ann Surg 1904; 40(1): 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorlund J B, Pihl K, Nissen N, Jørgensen U, Fristed J V, Lohmander L S, et al. Conundrum of mechanical knee symptoms: signifying feature of a meniscal tear? Br J Sports Med 2019; 53(5): 299–303. [DOI] [PubMed] [Google Scholar]
- Tinetti M E, Fried T. The end of the disease era. Am J Med 2004; 116(3): 179–85. [DOI] [PubMed] [Google Scholar]
- Van Arkel E R A, Koëter S, Rijk P C, Van Tienen T G, Vincken P W J, Segers M J M, et al. Dutch Guideline on Knee Arthroscopy Part 1, the meniscus: a multidisciplinary review by the Dutch Orthopaedic Association. Acta Orthop 2021; 92(1): 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Graaf V A, Noorduyn J C A, Willigenburg N W, Butter I K, de Gast A, Mol B W, et al. Effect of early surgery vs physical therapy on knee function among patients with nonobstructive meniscal tears: the ESCAPE randomized clinical trial. JAMA 2018; 320(13): 1328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Water R B, Leichtenberg C S, Nelissen R G H H, Kroon H M, Kaptijn H H, Onstenk R, et al. Preoperative radiographic osteoarthritis severity modifies the effect of preoperative pain on pain/function after total knee arthroplasty: results at 1 and 2 years postoperatively. J Bone Joint Surg Am 2019; 101(10): 879–87. [DOI] [PubMed] [Google Scholar]
- Wilson A S, Legg P G, McNeur J C. Studies on the innervation of the medial meniscus in the human knee joint. Anat Rec 1969; 165(4): 485–91. [DOI] [PubMed] [Google Scholar]
- Wynn Parry C B, Nichols P J, Lewis N R. Meniscectomy: a review of 1,723 cases. Ann Phys Med 1958; 4(6): 201–15. [DOI] [PubMed] [Google Scholar]
- Xiao M, Donahue J, Safran M R, Sherman S L, Abrams G D. Administrative databases utilized for sports medicine research demonstrate significant differences in underlying patient demographics and resulting surgical trends. Arthroscopy 2021; 37(1): 282–9.e1 [DOI] [PubMed] [Google Scholar]


