Figure 2.
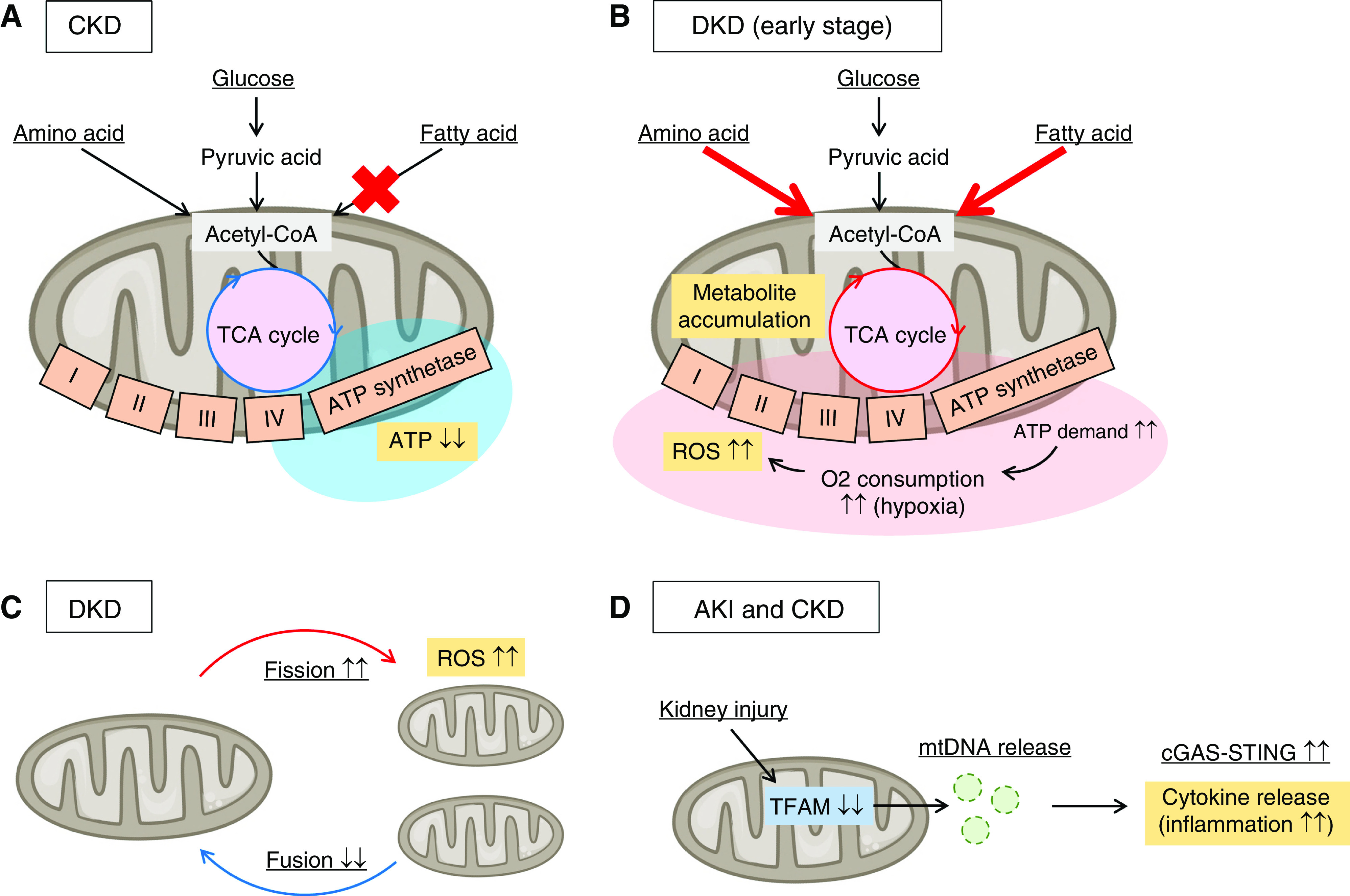
Mitochondrial stress responses are deeply involved in kidney disease progression. (A) Energy metabolism alterations of proximal tubules in CKD are shown. Defective fatty acid β-oxidation and mitochondrial dysfunction are observed in fibrotic kidneys, leading to ATP shortage. (B) Energy metabolism alterations of proximal tubules in the early stage of diabetic kidney disease (DKD) are shown. Mitochondrial respiration (mainly fueled by fatty acids and amino acids) is forcibly activated to meet the energy demand for glucose reabsorption in the hyperglycemic state, which increases oxygen consumption and results in renal hypoxia. Mitochondrial respiration in the hypoxic state produces a large amount of reactive oxygen species (ROS). (C) The imbalance in mitochondrial fission and fusion is observed in DKD. The increase in mitochondrial fission results in ROS overproduction. (D) Mitochondrial defects, including the loss of mitochondrial transcription factor A (TFAM) in proximal tubular cells, induce translocation of mitochondrial DNA (mtDNA) to the cytosol, which activates the innate immune pathway, cGAS-STING. This inflammatory response is observed both in AKI and CKD. O2, oxygen.
