Abstract
Kidney tissue hypoxia is detected in various kidney diseases and is considered to play an important role in the pathophysiology of both AKI and CKD. Because of the characteristic vascular architecture and high energy demand to drive tubular solute transport, the renal medulla is especially prone to hypoxia. Injured kidneys often present capillary rarefaction, inflammation, and fibrosis, which contribute to sustained kidney hypoxia, forming a vicious cycle promoting progressive CKD. Hypoxia-inducible factor (HIF), a transcription factor responsible for cellular adaptation to hypoxia, is generally considered to protect against AKI. On the contrary, consequences of sustained HIF activation in CKD may be either protective, neutral, or detrimental. The kidney outcomes seem to be affected by various factors, such as cell types in which HIF is activated/inhibited, disease models, balance between two HIF isoforms, and time and methods of intervention. This suggests multifaceted functions of HIF and highlights the importance of understanding its role within each specific context. Prolyl-hydroxylase domain (PHD) inhibitors, which act as HIF stabilizers, have been developed to treat anemia of CKD. Although many preclinical studies demonstrated renoprotective effects of PHD inhibitors in CKD models, there may be some situations in which they lead to deleterious effects. Further studies are needed to identify patients who would gain additional benefits from PHD inhibitors and those who may need to avoid them.
Keywords: chronic kidney disease, acute kidney injury, anemia, fibrosis, hypoxia, inflammation, microvascular rarefaction, prolyl-hydroxylase inhibitors, renal insufficiency, chronic, transcription factors
Introduction
Kidney tissue hypoxia is detected in various kidney diseases and is considered to contribute to the pathophysiology of both AKI and CKD. The pathological role of hypoxia-inducible factor (HIF), a master regulator of oxygen homeostasis, has been extensively studied, but it seems to be largely context dependent. Previous studies revealed that the kidney consequences of HIF activation/inhibition varied, depending on HIF isoforms, cell types, disease models, and time and methods of intervention. Now that HIF stabilizers have been introduced to treat anemia of CKD, there is a growing need to elucidate the specific role of HIF in each form of AKI and CKD. This review presents some key aspects of oxygen biology in the kidney, emphasizing the complex and multifaceted functions of HIF and the importance of understanding its pathological role in each disease condition.
Susceptibility of the Kidney to Hypoxia
In 1960, Aukland and Krog (1) reported heterogeneous oxygen tension within the kidney of healthy dogs, which was much lower in the medullary region compared with the cortex. The subsequent studies also reported that oxygen tension in the medulla was 10–20 mm Hg, whereas that in the cortex was 30–60 mm Hg (2–4). However, it was later recognized that the tissue oxygenation was affected by general anesthesia, which reduces renal blood flow (6 Nephrectomy). The medullary oxygen tension of healthy, nonanesthetized sheep was reported to be 30–40 mm Hg, which was similar to that in the cortex (6). It should be noted, however, that the basal tissue perfusion in the medulla was demonstrated to be significantly less, and the decrease in perfusion and oxygenation was greater in the medullary region during partial renal artery occlusion (6). This finding suggests that its inadequate ability to maintain oxygen homeostasis renders the renal medulla particularly susceptible to hypoxia under pathological conditions. In addition, studies using a hypoxic marker, pimonidazole, demonstrated positive staining in the medulla and corticomedullary regions in kidneys of healthy rats (7,8). These observations led to the concept that the renal medulla is “relatively hypoxic,” even if there is no apparent decrease in tissue oxygen tension.
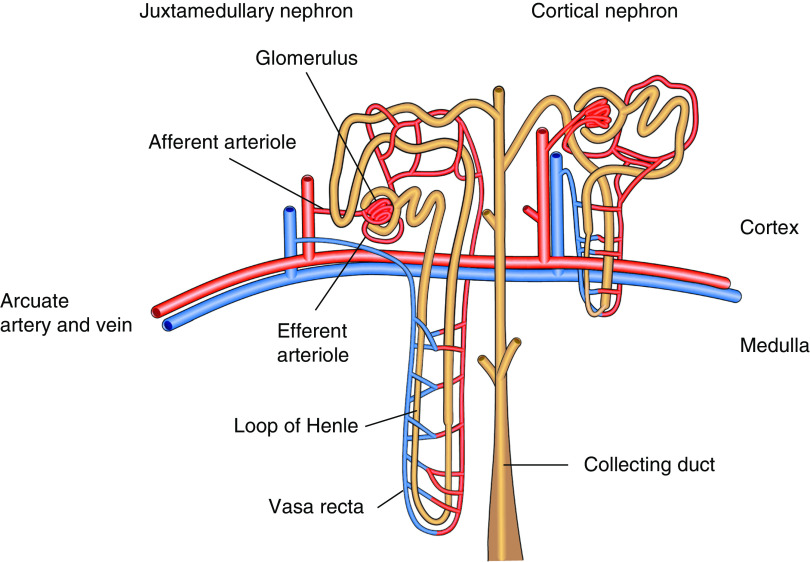
This susceptibility of the renal medulla to hypoxia is considered to arise from the characteristic vascular architecture of the kidney (9,10). The efferent arterioles of the cortical nephrons give rise to the peritubular capillaries that perfuse proximal and distal tubules in the cortex, whereas those that arise from juxtamedullary nephrons form the vasa recta, which run in parallel with the loops of Henle and collecting ducts (Figure 1). Due to this vascular architecture, the renal medulla receives only 6 Nephrectomy%–10% of the total renal blood flow (11). There is a diffusive oxygen shunt between arterial and venous vessels in the vasa recta, called the arteriovenous oxygen shunt, further limiting the oxygen availability in the renal medulla (12,13). Despite this inefficient oxygen delivery, the tubular cells have high metabolic demands to drive solute transport, which makes them especially vulnerable to hypoxic stress (14).
Figure 1.
The renal medulla is prone to hypoxia due to the characteristic vascular atchitecture of the kidney. The efferent arterioles of the cortical nephrons give rise to the peritubular capillaries, which perfuse proximal and distal tubules in the cortex, whereas those that arise from juxtamedullary nephrons form the vasa recta, which run in parallel with the loops of Henle and collecting ducts.
Methods To Detect Hypoxia in the Kidney
There are several methods to measure oxygen tension in the kidney (Table 1). Polarographic oxygen microelectrodes have been the gold standard in animal experiments and have provided much of our knowledge regarding kidney oxygenation (1–4). The recent development of implantable microelectrodes in combination with telemetry devices has enabled the continuous monitoring of kidney oxygenation without the influence of anesthesia (,16 Nephrectomy). Microelectrodes make use of oxidation-reduction reactions, whereas another method uses oxygen quenching of fluorescence on the basis that the fluorescence lifetime is inversely proportional to oxygen tension (16). The direct comparison of the oxygen tension measured by these two methods revealed the value obtained by the fluorescence optode tended to be lower than that obtained by the microelectrode (17). Nevertheless, both measurements reflected the changes in inspired oxygen concentration and arterial blood oxygen levels (17), indicating that both methods are equally applicable to experiments that evaluate acute changes in kidney tissue oxygenation under pathological conditions.
Table 1.
Methods to detect kidney hypoxia
| Methods | Data Type | Strengths | Limitations |
| Polarographic microelectrodes | Quantitative | Has been well established and has a substantial amount of data | Difficult to obtain oxygen tension over large areas |
| Can measure both cortical and medullary oxygen tension by adjusting the depth of the sensor | Tissue damage due to the insertion of sensors | ||
| Continuous telemetric measurement has been developed | Oxygen consumption during measurement | ||
| Fluorescence optodes | Quantitative | Greater accuracy at low oxygen tension compared to microelectrodes | Difficult to obtain oxygen tension over large areas |
| No oxygen consumption at the sensor tip | Tissue damage due to the insertion of sensors (the sensor is larger than microelectrodes) | ||
| Phosphorescence lifetime measurement | Quantitative | Can assess intracellular oxygenation if appropriate dye is used | Cannot measure oxygen tension in deeper parts of the kidney |
| Pimonidazole immunohistochemistry | Qualitative | Provides spatial resolution of hypoxic areas | Assessment of hypoxia only at a discrete time point |
| Possibility of false positive staining/hypoxia may be introduced during tissue harvest | |||
| BOLD-MRI | Semiquantitative | Noninvasive and repeatable | Moderate spatial resolution |
| Can be used in humans | The R2* values can be influenced by internal and external factors | ||
| Oxygen mapping of the entire kidney is available | Image analysis can be difficult | ||
| Urine oximetry | Quantitative | Noninvasive and applicable to clinical settings | Confounding factors could influence the results |
| May be used as a biomarker for postoperative AKI | Limited evidence at present |
BOLD-MRI, blood oxygen level–dependent magnetic resonance imaging.
Phosphorescence lifetime measurement is another method recently developed to assess kidney oxygenation (18). Similar to the fluorescence optode, this method is based on oxygen-dependent quenching of luminescence. The lifetime of phosphorescence is longer, and this gives an advantage in the avoidance of confounding effects of autofluorescence (19). This method requires administration of a phosphorescent dye, and the development of dyes with increased cellular uptake has enabled intracellular oxygen sensing in living cells and animals (20). One of such dyes, BTPDM1, was demonstrated to distribute inside tubular cells of mouse kidney, and phosphorescence lifetime measurement of BTPDM1 detected hypoxia in tubular cells during renal artery clamping (21). When combined with a confocal microscopy, this method is able to provide a high-spatial-resolution image of kidney oxygen tension, and it showed for the first time that the intracellular oxygen tension varied across tubular epithelial cells in the superficial cortex (22).
Apart from these electrochemical and optical methods, pimonidazole immunohistochemistry is frequently used to detect hypoxia in the kidney. Pimonidazole is a 2-nitroimidazole that is reductively activated in hypoxic cells and forms stable adducts with thiol-containing proteins (23). For in vivo assessment of hypoxia, pimonidazole is administered to animals before tissue harvest and the protein adducts are detected using immunohistochemistry, which clearly distinguishes hypoxic areas. Positive staining is often observed in the medulla and corticomedullary regions under physiologic conditions (7,8), and hypoxic areas extend to the cortex in CKD and aging kidneys (24,26 Nephrectomy).
Blood oxygen level–dependent magnetic resonance imaging (BOLD-MRI) is increasingly used to assess kidney oxygenation due to its noninvasiveness and applicability to humans. BOLD-MRI measures the R2* value, which is proportional to the blood content of deoxyhemoglobin (26). Experimental evidence demonstrated a linear relationship between the R2* values and the oxygen tension measured by microelectrodes (27). It should be noted, however, that the R2* values are influenced by several factors, such as hydration status and dietary salt intake (28,29). In addition, there are four major methods to analyze BOLD-MRI images, each with their own strengths and weaknesses (30). International effort is now being made to standardize the BOLD-MRI protocols, including patient preparation, image acquisition, and analysis (30,31).
Urine oximetry is another method that has a potential to be used in clinical settings. The urinary oxygen tension is measured with either polarographic electrodes or fluorescence optodes equipped in bladder catheters (32,33), and it was shown to reflect medullary tissue oxygenation (33). Zhu et al. (34) measured urinary oxygen tension in patients who underwent cardiac surgery that required cardiopulmonary bypass. They found the oxygen tension was lower in patients who later developed AKI, suggesting that urinary hypoxia may be a useful predictor of postoperative AKI.
Hypoxia in AKI and CKD
Kidney tissue hypoxia has been detected in multiple forms of AKI, including postoperative AKI, sepsis, and drug-induced nephropathy (33–36 Nephrectomy). Experimental models of sepsis demonstrated a significant decrease in the proportion of peritubular capillaries that showed normal blood flow (36). The mechanism of this abnormal microcirculation is incompletely understood, but it has been proposed that increased production of inducible nitric oxide synthase and cytokine-induced endothelial damage may have played a role (37). In addition, unbalanced oxygen supply and demand also contributes to the development of hypoxia in AKI. For example, administration of radiocontrast agents increased oxygen consumption for tubular transport, and inhibition of transport activity with furosemide reversed contrast agent–induced medullary hypoxia (38). It must be noted, however, that solely improving medullary oxygenation by furosemide does not necessarily translate into better kidney outcomes (39).
AKI is now recognized as an independent risk factor for CKD, i.e., patients with a history of AKI are more likely to develop CKD, even if they make a complete recovery of kidney function. Incomplete or maladaptive repair of peritubular capillaries and tubular epithelium generates persistent kidney hypoxia, which contributes to the AKI-to-CKD transition (40). Decreased vascular density and increased pimonidazole-stained area were observed 6 Nephrectomy weeks after ischemia-reperfusion injury in rats, although serum creatinine had returned to the basal level (41). Administration of l-arginine increased renal blood flow and improved kidney oxygenation in these rats, preventing progressive decline in creatinine clearance during the 20-week observation period after the injury (41). This result clearly shows that tissue hypoxia due to capillary rarefaction contributes to the AKI-to-CKD transition. The loss of peritubular capillaries may be explained, in part, by a decrease in vascular endothelial growth factor secreted by tubular cells (42,43). It was suggested that reduced expression of vascular endothelial growth factor was a characteristic of tubular cells that failed to completely redifferentiate after acute injury (43). These malfunctioning tubules secrete profibrotic factors, such as connective tissue growth factor, PDGF-B, and TGF-β, which promote kidney fibrosis (44). Interstitial fibrosis inhibits oxygen diffusion between peritubular capillaries and parenchyma, and the resultant kidney tissue hypoxia further accelerates fibrogenesis, forming a vicious cycle promoting progression from AKI to CKD (9).
In addition to the maladaptive repair of peritubular capillaries and tubular epithelium, AKI episodes, especially hypoxic insults, induce epigenetic changes that promote proinflammatory and profibrotic gene expression. Hypoxia-induced epigenetic changes include DNA methylation, histone modification, chromatin conformational changes, and altered expression of noncoding RNAs (,46 Nephrectomy,46). Zager et al. (47) detected increased levels of gene-activating histone modifications (H3K4me3 and H2A.Z) after ischemia-reperfusion injury, which corresponded with increases in monocyte chemoattractant protein-1 and TGF-β expressions. These persistent epigenetic changes serve as “hypoxic memory” and contribute to the AKI-to-CKD transition in the long term after recovery from the initial AKI episode. Pharmacological inhibition of a histone methyltransferase suppressed kidney fibrosis 8 weeks after ischemia-reperfusion injury, indicating therapeutic potential of epigenetic interventions (48).
Kidney hypoxia is also detected in various forms of CKD that do not present apparent episodes of AKI, such as those related to diabetes and hypertension (49,6 Nephrectomy0). As in the AKI-to-CKD transition, capillary rarefaction, interstitial fibrosis, and inflammation are critical contributors to kidney hypoxia. Although it is difficult to firmly establish that hypoxia per se promotes progression of CKD, a study using BOLD-MRI demonstrated that low cortical oxygenation was indeed an independent predictor of kidney function decline (6 Nephrectomy1,6 Nephrectomy2). The importance of hypoxia in the course of CKD was also highlighted in a study that used dinitrophenol, a mitochondrial uncoupler. Administration of dinitrophenol in rats increased renal oxygen consumption and reduced oxygen tension in both the cortex and medulla. Treatment with dinitrophenol for 30 days increased urinary protein excretion, tubular damage, and infiltration of inflammatory cells. These findings indicated that kidney tissue hypoxia, by itself, was sufficient to trigger kidney injury (6 Nephrectomy3).
Hypoxia-Inducible Transcription Factors
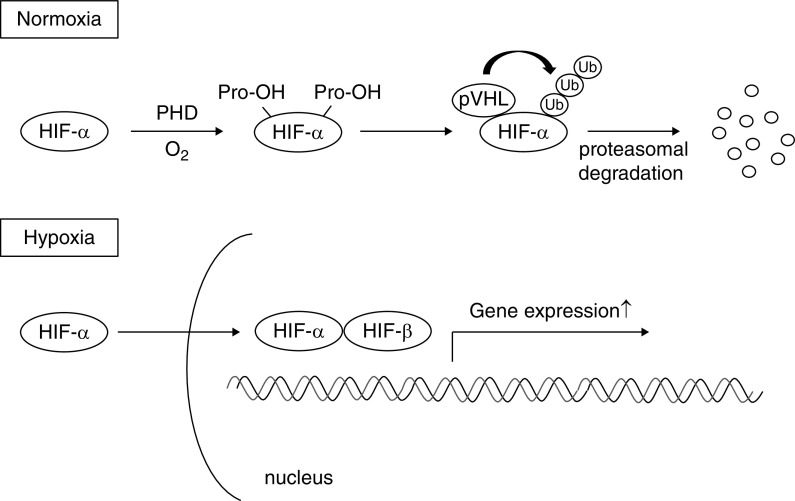
Kidney cells, as well as other cell types in the body, have evolved mechanisms of hypoxic adaptation. The most important player in this system is HIF, a transcription factor responsible for induction of genes essential for survival under hypoxic conditions. HIF is a heterodimer composed of a constitutively expressed β subunit and an oxygen-regulated α subunit. The α subunits are synthesized continuously, irrespective of the oxygen status of the cells. Under normoxic conditions, prolyl-hydroxylase domain (PHD)–containing proteins hydroxylate specific proline residues of HIF-α. Proline-hydroxylated HIF-α is recognized by the von Hippel–Lindau (VHL)–E3 ubiquitin ligase complex, resulting in HIF-α ubiquitination and subsequent proteasomal degradation. Under hypoxic conditions, hydroxylation of HIF-α is inhibited, allowing translocation to the nucleus where it dimerizes with HIF-β and binds to the hypoxia-response element, inducing transcription of target genes (Figure 2) (54–56).
Figure 2.
Prolyl-hydroxylase domain–containing protein regulates the stability of hypoxia-inducible factor according to the oxygen availability. Under normoxic conditions, hypoxia-inducible factor-α (HIF-α) is hydroxylated by prolyl-hydroxylase domain (PHD) proteins. Hydroxylated HIF-α is recognized by the von Hippel–Lindau- E3 ubiquitin ligase complex, resulting in HIF-α ubiquitination and subsequent proteasomal degradation. Under hypoxic conditions, hydroxylation of HIF-α is inhibited, allowing translocation to the nucleus where it dimerizes with HIF-β and binds to the hypoxia-response element, inducing transcription of target genes. O2, oxygen; Pro-OH, hydroxylated proline residue; pVHL, von Hippel-Lindau protein; Ub, ubiquitin.
Mammals have three principal isoforms of HIF-α, HIF-1α, HIF-2α, and HIF-3α, which dimerize with HIF-β to form HIF-1, HIF-2, and HIF-3, respectively. HIF-1α and HIF-2α have a similar domain architecture and undergo similar proteolytic regulation, but have different expression patterns and partly overlapping, but largely nonredundant, functions. In ischemic kidney, HIF-1α is expressed predominantly in tubular cells, whereas HIF-2α is expressed mainly in endothelial and interstitial cells (57). The role of HIF-3α is not yet fully understood. There are also three isoforms of PHD enzymes, PHD1, PHD2, and PHD3, among which PHD2 is the major regulator of HIF activity (,54,58).
HIF was first discovered in 1992 in the effort to unveil the regulatory mechanism of erythropoietin (EPO) production (59), which had been demonstrated to increase in rodent kidney in response to hypoxia or anemia (60,61). Arterial oxygen content, determined by hemoglobin concentration, arterial oxygen saturation, and arterial oxygen tension, is the major determinant of the amount of EPO produced (62). Both reduced arterial oxygen content and reduced renal blood flow decrease kidney tissue oxygenation, which subsequently activates HIF-2 and induces transcription of EPO in peritubular interstitial fibroblast-like cells (63). The discovery of this regulatory mechanism led to the development of small-molecule PHD inhibitors to treat anemia of CKD, which promote endogenous EPO production by activating HIF (63,64). Furthermore, this oxygen-sensing system turned out to be universal, regulating a broad spectrum of genes essential for oxygen homeostasis in virtually all mammalian cells. The PHD-HIF system is also involved in the pathophysiology of various diseases, including cancer, inflammation, cardiovascular diseases, and kidney diseases (65). Because of the important implications of their work, the Nobel Prize in Physiology or Medicine 2019 was awarded to three scientists, Gregg L. Semenza, Peter J. Ratcliffe, and William G. Kaelin Jr., who discovered HIF and unveiled its oxygen-dependent regulatory mechanism (66).
The Effects of HIF Activation in AKI
It is generally accepted that HIF activation protects against AKI. We demonstrated first in 2003 that administration of cobalt chloride, which inhibits PHD activity and stabilizes HIF, reduced tubulointerstitial injury after ischemia reperfusion (67). Other HIF stabilizers, such as carbon monoxide, xenon, and small-molecule PHD inhibitors had similar effects (68,69). Systemic deletion of Vhl, which resulted in activation of HIF-1 and HIF-2, also ameliorated tubular injury and kidney dysfunction in the same AKI model (70). Conversely, both heterozygous Hif1α and Hif2α knockdown mice presented more profound ischemia-reperfusion injury compared with their wild-type littermates (71). We also demonstrated that systemic knockdown of Hif2α alone was sufficient to aggravate tubulointerstitial injury, and restoration of Hif2α in endothelial cells ameliorated kidney damage after ischemia-reperfusion injury (72). Similar protective effects of HIF were observed in AKI of other etiologies, including cisplatin-induced nephropathy and graft injury during transplantation (73,74). Although it is difficult to elucidate the exact mechanism of renoprotection, HIF activation is often associated with a reduction in tubular cell apoptosis, infiltration of inflammatory cells, and peritubular capillary loss (67–74). A recent study demonstrated that HIF-induced glycogen synthesis contributed to cell survival under oxygen-glucose deprivation (75). It should be emphasized, however, that all of the above studies took preventive strategies, i.e., HIF was activated before the insults (67–75). In fact, administration of PHD inhibitors after ischemia-reperfusion injury failed to ameliorate tubulointerstitial damage (76,77), suggesting a time-related therapeutic window for optimal effects of HIF activation in the course of AKI.
The Effects of HIF Activation in CKD
HIF accumulation has been observed in multiple forms of CKD (78–80), and there is much debate about its pathophysiological role. Although HIF is generally considered to promote cell survival under hypoxic conditions, some studies suggest that long-term HIF activation may bring about its harmful effects, such as fibrogenesis and inflammation. For example, HIF was demonstrated to induce profibrotic factors (81), and stabilization of HIF in kidney proximal tubules, by genetic deletion of Vhl, promoted tubulointerstitial fibrosis in a subtotal nephrectomy model (82). An opposing view is that HIF activation in CKD is in fact insufficient to achieve optimal cytoprotection. This notion is supported by studies that demonstrated the effects of indoxyl sulfate, a representative uremic toxin, on HIF activity. Administration of indoxyl sulfate suppressed nuclear accumulation of HIF-2α and subsequent production of EPO (83). Indoxyl sulfate also inhibited HIF-1 activity by inducing the expression of transcriptional repressors of HIF-1 (84). Additionally, oxidative stress and the diabetic milieu impair HIF functions (78,85,86). These findings led to the idea that therapeutic strategy to activate HIF may facilitate adaptive response to hypoxia and prevent CKD progression.
Numerous preclinical studies have been conducted to investigate the consequences of HIF activation/inhibition in CKD, but they present controversial results (Tables 2 and 3). These controversies may arise from differences in the methods of intervention, including specificity of the compounds used, disease models, manipulated HIF isoforms, and cell types involved, reflecting the multifaceted functions of HIF and its complex regulatory system. By and large, systemic administration of PHD inhibitors demonstrated renoprotection in CKD: pharmacological HIF activation alleviated tubulointerstitial injury in 5/6 nephrectomy (87), Thy-1 nephritis (88), streptozotocin-induced diabetes (89), and adenine-induced nephropathy (90). Global activation of HIF by genetic deletion of Vhl also ameliorated kidney fibrosis and macrophage infiltration in a unilateral ureteral obstruction (UUO) model (91). In contrast, local deletion of Vhl by the γ-glutamyl transpeptidase promoter–driven Cre recombinase, which resulted in HIF-1 activation in proximal tubular epithelial cells, exacerbated fibrosis after 5/6 nephrectomy (82). Along the same lines, proximal tubule–specific deletion of Hif1α by phosphoenolpyruvate carboxykinase–driven Cre recombinase ameliorated fibrosis in the UUO kidney (92).
Table 2.
Preclinical studies to investigate the effects of PHD inhibitors on the progression of various models of CKD
| CKD Model | Approach for HIF Activation/Inhibition | Which HIF Was Activated/Inhibited | Outcomes | HIF Protective or Deleterious | Reference |
| 5/6 Nephrectomy | Cobalt for 4 wk, starting 5 wk after the surgery | HIF-1 and HIF-2 activated | Tubulointerstitial injury ↓, serum creatinine ↓ | Protective | (87) |
| Uninephrectomized Thy-1 nephritis | Cobalt for 3 wk, starting a wk after Thy-1 injection | HIF-1 and HIF-2 activated | Tubulointerstitial injury ↓ | Protective | (88) |
| Glomerular injury was not affected | |||||
| STZ-induced diabetes | Cobalt for 4 wk, starting right after the induction of diabetes | HIF-1 and HIF-2 activated | Tubulointerstitial injury ↓, proteinuria ↓ | Protective | (89) |
| Adenine-induced nephropathy | PHD inhibitors (ICA or roxadustat) for 3 wk, coadministered with adenine | HIF-1 and HIF-2 activated | Proteinuria ↓, plasma creatinine ↓, tubulointerstitial damage ↓, fibrosis → | Protective | (90) |
| 5/6 Nephrectomy | (1) l-Mimosine from 2 to 12 wk after the surgery | HIF-1 and HIF-2 activated | (1) Exacerbated glomerular and tubulointerstitial injury | Depends on timing | (95) |
| (2) l-Mimosine from 4 to 12 wk after the surgery | (2) Ameliorated glomerular and tubulointerstitial injury | ||||
| (3) l-Mimosine from 8 to 12 wk after the surgery | (3) No effects |
PHD, prolyl-hydroxylase domain; HIF, hypoxia-inducible factor; STZ, streptozotocin; ICA, 2-(1-chloro-4-hydroxy isoquinoline-3-carboxamido) acetate.
Table 3.
Preclinical studies to investigate the effects of HIF-related gene manipulation on the progression of various models of CKD
| CKD Model | Approach for HIF Activation/Inhibition | Which HIF Was Activated/Inhibited | Outcomes | HIF Protective or Deleterious | Reference |
| 5/6 Nephrectomy | γ -GT-Cre Vhl−/− | Theoretically, both HIF-1 and HIF-2 were activated in proximal tubular cells | Fibrosis ↑ | Deleterious | (82) |
| (Only HIF-1α accumulation was shown) | Vhl−/− mice exhibited more profound fibrosis at the age of 60 wk. | ||||
| UUO | PEPCK-Cre Hif1α−/− | HIF-1 was inhibited in proximal tubular cells | Epithelial-to-mesenchymal transition ↓, fibrosis ↓ | Deleterious | (92) |
| UUO | (1) Ubc-Cre Vhl−/− | (1) Global activation of HIF-1 and HIF-2 | (1) Fibrosis ↓, macrophage infiltration ↓ | Protective | (91) |
| (2) Ubc-Cre Hif−/− | (2) Global inhibition of HIF-1 and HIF-2 | (2) Fibrosis →, macrophage infiltration ↑ | |||
| (3) LysM-Cre Vhl−/− | (3) Myeloid cell–specific activation of HIF-1 and HIF-2 | (3) Fibrosis →, macrophage infiltration ↓ | |||
| (4) LysM-Cre Hif−/− | (4) Myeloid cell–specific inhibition of HIF-1 and HIF-2 | (4) Fibrosis →, macrophage infiltration ↑ | |||
| Anti-GBM GN | Pax8-rtTA Vhl−/− | HIF-1 and HIF-2 were activated in tubular cells | Glomerular and tubular injury ↓, plasma urea ↓, proteinuria ↓ | Protective | (93) |
| ADPKD (Ksp-Cre Pdk1−/−) | (1) Ksp-Cre Hif-1α−/− | (1) HIF-1 inhibition in tubular cells | (1) Cyst growth ↓ | Deleterious | (101) |
| (2) PHD inhibitor | (2) Global activation of HIF-1 and HIF-2 | (2) Cyst growth ↑ and earlier death | |||
| High-fat diet | Ndrg1-Cre-Phd2−/− | Proximal tubule–specific HIF activation | Tubular damage ↓, albuminuria ↓, glomerulomegaly ↓ | Protective | (94) |
HIF, hypoxia-inducible factor; γ-GT, γ-glutamyl transpeptidase; UUO, unilateral ureteral obstruction; PEPCK, phosphoenolpyruvate carboxykinase; Ubc, ubiquitin C; LysM, lysin motif; GBM, glomerular basement membrane; Pax8-rtTA, Pax8-reverse tetracycline–dependent transactivator; ADPKD, autosomal dominant polycystic kidney disease; Ksp, kidney-specific cadherin; PHD, prolyl-hydroxylase domain; Ndrg1, N-myc downstream regulated gene 1.
These seemingly contradicting results may be explained, in part, by the cell type–specific functions of HIF, because systemic administration of PHD inhibitors not only activates HIF in tubular epithelium, but also in other intra- and extrarenal cells. For example, a study demonstrated that myeloid-specific inactivation of Hif1α and Hif2α increased macrophage infiltration in a UUO model, suggesting a critical role of HIF in myeloid cells in regulating kidney inflammation (91). Similarly, depletion of mononuclear phagocytes with clodronate largely abolished the protective effect of a PHD inhibitor, although this study also demonstrated that neither HIF-1α nor HIF-2α in myeloid cells was required for kidney protection, suggesting some other HIF-independent mechanisms (90).
However, it would be premature to conclude that HIF activation in proximal tubules is deleterious in CKD. Pax8-reverse tetracycline–dependent transactivator–mediated inactivation of Vhl induced nuclear accumulation of HIF-1α and HIF-2α in tubular epithelial cells, and it ameliorated both glomerular and tubulointerstitial injury in an antiglomerular basement membrane GN model (93). Likewise, mice with proximal tubule–specific knockout of Phd2 by N-myc downstream regulated gene 1 promoter-driven Cre recombinase were protected against kidney injury induced by a high-fat diet (94). The results of the studies using genetically modified animals seem to be affected by the following factors: the cell type–specific promoter used in gene manipulation, the gene (Vhl, Hif1α, Hif2α, or Phd) targeted, and the disease model used. HIF is likely to have a highly context-dependent function, and the effects observed in one study may not be evident in other situations.
In addition to cell types and disease models, the timing of HIF activation/inhibition also affects the experimental outcomes. Yu et al. (95) performed 5/6 nephrectomy in rats and treated them with a PHD inhibitor, l-mimosine, from 2 or 4 weeks after the surgery. They found that the rats treated early had more profound glomerular and tubulointerstitial injury, whereas those treated from 4 weeks presented milder injury compared with the control animals (95). The final nuclear expression levels of HIF-1α and HIF-2α were different between these two groups, suggesting the balance between HIF-1 and HIF-2 may have influenced the progression of CKD (95). In the case of lung epithelial cells, HIF-1α was demonstrated to decrease under prolonged hypoxia, whereas HIF-2α remained upregulated (96). Similar temporal specificity of HIF isoforms might exist during the course of CKD.
Pleiotropic Effects of PHD Inhibitors
Small-molecule PHD inhibitors have been developed to treat anemia of CKD and roxadustat has been already launched in China and Japan (63,64). They promote endogenous EPO production by activating HIF. However, considering the broad spectrum of genes regulated by PHD-HIF system, it would not be surprising if PHD inhibitors have additional effects other than erythropoiesis. One possible benefit of PHD inhibitors is their potential to protect against obesity and metabolic disorders. A preclinical study demonstrated that systemic Phd2-hypomorphic mice were resistant to high-fat-diet-induced obesity and glucose intolerance (97). Likewise, administration of a PHD inhibitor, enarodustat, ameliorated insulin resistance and decreased albuminuria in obese type 2 diabetic mice, which was associated with reduced glomerular monocyte chemoattractant protein-1 expression and less macrophage infiltration. Enarodustat also maintained plasma adiponectin levels, which may have contributed to kidney protection (98). Furthermore, transomics approaches using transcriptome and metabolome analyses revealed that, in the early stage of experimental type 1 diabetes, a PHD inhibitor counteracted diabetic renal metabolisms of fatty acids and amino acids, which were upregulated in the diabetic kidney and downregulated by a PHD inhibitor. These changes were associated with less accumulation of glutathione disulfide, thus increasing the glutathione/glutathione disulfide ratio and ameliorating glomerular hypertrophy (99). On the other hand, PHD inhibitors may have deleterious effects. For example, FG-4592/roxadustat was shown to promote phosphate-induced vascular smooth muscle cell calcification in vitro (100), which may lead to atherosclerosis. Other concerns include theoretical risks for tumor growth, pulmonary hypertension, and angiogenesis which may facilitate the progression of diabetic retinopathy and age-related macular degeneration (64). Although many preclinical studies demonstrated renoprotective effects in various CKD models, caution may be needed in autosomal dominant polycystic kidney disease. HIF-1α was shown to accelerate cyst growth, and administration of a PHD inhibitor promoted cyst expansion and kidney dysfunction in the mouse model of autosomal dominant polycystic kidney disease (101). This effect was mediated by increased calcium-dependent chloride secretion (102), suggesting that some parts of the HIF-activated pathways may bring about deleterious effects under certain circumstances.
Several caveats exist in translating findings obtained using transgenic/knockout mice into pharmacological PHD inhibition. First, small-molecule PHD inhibitors distribute preferentially in organs such as the liver and kidney, and the local effects are not likely to be as uniform as that accomplished in genetic studies. Second, the levels of achieved HIF-α accumulation are not likely to be similar among methods for intervention. For example, Vhl knockout, which is in many cases used as genetic manipulation to activate HIF, results in by far the most robust accumulation of HIF-1α and HIF-2α, as compared with Phd2 knockout, in many organs including the kidney (103). In the case of PHD inhibitors, the levels of HIF-α are most likely to fluctuate within dosing intervals, as envisaged by plasma EPO levels peaking at 8–9 hours after single administration of roxadustat (104). Overall, findings obtained from genetic studies may not be the same as those expected in PHD inhibitors. Further studies are needed to address potential applicability in human clinical settings.
Conclusions
Although it is generally accepted that HIF activation protects against AKI, controversial results have been reported for CKD. The final outcomes are affected by various factors, such as balance between two HIF isoforms, cell types, disease models, and time and methods of intervention. This suggests that HIF has multifaceted functions, and it is important to understand its pathological role within each specific context. PHD inhibitors may promote or delay the progression of CKD, depending on its etiology, CKD stages, and comorbidities. Further studies are needed to identify patients who would gain additional benefits from PHD inhibitors and those who may need to avoid them.
Disclosures
M. Nangaku has received honoraria and research grants from Akebia, Astellas, Bayer, GSK, JT, Kyowa-Kirin, and Mitsubishi Tanabe. T. Tanaka has received honoraria from Kyowa-Kirin and a research grant from JT, outside of the submitted work. The remaining author has nothing to disclose.
Funding
None.
Author Contributions
M. Sugahara wrote the original draft; M. Nangaku and T. Tanaka reviewed and edited the manuscript.
References
- 1.Aukland K, Krog J: Renal oxygen tension. Nature 188: 671, 1960 [DOI] [PubMed] [Google Scholar]
- 2.Brezis M, Heyman SN, Dinour D, Epstein FH, Rosen S: Role of nitric oxide in renal medullary oxygenation. Studies in isolated and intact rat kidneys. J Clin Invest 88: 390–395, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brezis M, Heyman SN, Epstein FH: Determinants of intrarenal oxygenation. II. Hemodynamic effects. Am J Physiol 267: F1063–F1068, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Brezis M, Agmon Y, Epstein FH: Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol 267: F1059–F1062, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Groves ND, Leach KG, Rosen M: Effects of halothane, enflurane and isoflurane anaesthesia on renal plasma flow. Br J Anaesth 65: 796–800, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Calzavacca P, Evans RG, Bailey M, Lankadeva YR, Bellomo R, May CN: Long-term measurement of renal cortical and medullary tissue oxygenation and perfusion in unanesthetized sheep. Am J Physiol Regul Integr Comp Physiol 308: R832–R839, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Rosenberger C, Rosen S, Paliege A, Heyman SN: Pimonidazole adduct immunohistochemistry in the rat kidney: Detection of tissue hypoxia. Methods Mol Biol 466: 161–174, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Manotham K, Tanaka T, Matsumoto M, Ohse T, Miyata T, Inagi R, Kurokawa K, Fujita T, Nangaku M: Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol 15: 1277–1288, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Mimura I, Nangaku M: The suffocating kidney: Tubulointerstitial hypoxia in end-stage renal disease. Nat Rev Nephrol 6: 667–678, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Cowley AW Jr.: Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol 273: R1–R15, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Levy MN, Sauceda G: Diffusion of oxygen from arterial to venous segments of renal capillaires. Am J Physiol 196: 1336–1339, 1959 [DOI] [PubMed] [Google Scholar]
- 13.Welch WJ, Baumgärtl H, Lübbers D, Wilcox CS: Nephron pO2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int 59: 230–237, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Evans RG, Harrop GK, Ngo JP, Ow CPC, O’Connor PM: Basal renal O2 consumption and the efficiency of O2 utilization for Na+ reabsorption. Am J Physiol Renal Physiol 306: F551–F560, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Koeners MP, Ow CPC, Russell DM, Abdelkader A, Eppel GA, Ludbrook J, Malpas SC, Evans RG: Telemetry-based oxygen sensor for continuous monitoring of kidney oxygenation in conscious rats. Am J Physiol Renal Physiol 304: F1471–F1480, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Evans RG, Gardiner BS, Smith DW, O’Connor PM: Methods for studying the physiology of kidney oxygenation. Clin Exp Pharmacol Physiol 35: 1405–1412, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Leong CL, O’Connor PM, Eppel GA, Anderson WP, Evans RG: Measurement of renal tissue oxygen tension: Systematic differences between fluorescence optode and microelectrode recordings in anaesthetized rabbits. Nephron, Physiol 108: p11-7, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Hirakawa Y, Tanaka T, Nangaku M: Renal hypoxia in CKD; Pathophysiology and detecting methods. Front Physiol 8: 99, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You Y, Lee S, Kim T, Ohkubo K, Chae WS, Fukuzumi S, Jhon GJ, Nam W, Lippard SJ: Phosphorescent sensor for biological mobile zinc. J Am Chem Soc 133: 18328–18342, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobita S, Yoshihara T: Intracellular and in vivo oxygen sensing using phosphorescent iridium(III) complexes. Curr Opin Chem Biol 33: 39–45, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Hirakawa Y, Yoshihara T, Kamiya M, Mimura I, Fujikura D, Masuda T, Kikuchi R, Takahashi I, Urano Y, Tobita S, Nangaku M: Quantitating intracellular oxygen tension in vivo by phosphorescence lifetime measurement. Sci Rep 5: 17838, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirakawa Y, Mizukami K, Yoshihara T, Takahashi I, Khulan P, Honda T, Mimura I, Tanaka T, Tobita S, Nangaku M: Intravital phosphorescence lifetime imaging of the renal cortex accurately measures renal hypoxia. Kidney Int 93: 1483–1489, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Arteel GE, Thurman RG, Raleigh JA: Reductive metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension independent of the pyridine nucleotide redox state. Eur J Biochem 253: 743–750, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto M, Tanaka T, Yamamoto T, Noiri E, Miyata T, Inagi R, Fujita T, Nangaku M: Hypoperfusion of peritubular capillaries induces chronic hypoxia before progression of tubulointerstitial injury in a progressive model of rat glomerulonephritis. J Am Soc Nephrol 15: 1574–1581, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Tanaka T, Kato H, Kojima I, Ohse T, Son D, Tawakami T, Yatagawa T, Inagi R, Fujita T, Nangaku M: Hypoxia and expression of hypoxia-inducible factor in the aging kidney. J Gerontol A Biol Sci Med Sci 61: 795–805, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Prasad PV: Evaluation of intra-renal oxygenation by BOLD MRI. Nephron Clin Pract 103: c58–c65, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Pedersen M, Dissing TH, Mørkenborg J, Stødkilde-Jørgensen H, Hansen LH, Pedersen LB, Grenier N, Frøkiaer J: Validation of quantitative BOLD MRI measurements in kidney: Application to unilateral ureteral obstruction. Kidney Int 67: 2305–2312, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Prasad PV, Epstein FH: Changes in renal medullary pO2 during water diuresis as evaluated by blood oxygenation level-dependent magnetic resonance imaging: Effects of aging and cyclooxygenase inhibition. Kidney Int 55: 294–298, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pruijm M, Hofmann L, Maillard M, Tremblay S, Glatz N, Wuerzner G, Burnier M, Vogt B: Effect of sodium loading/depletion on renal oxygenation in young normotensive and hypertensive men. Hypertension 55: 1116–1122, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Pruijm M, Mendichovszky IA, Liss P, Van der Niepen P, Textor SC, Lerman LO, Krediet CTP, Caroli A, Burnier M, Prasad PV: Renal blood oxygenation level-dependent magnetic resonance imaging to measure renal tissue oxygenation: A statement paper and systematic review. Nephrol Dial Transplant 33[Suppl 2]: ii22-ii28, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bane O, Mendichovszky IA, Milani B, Dekkers IA, Deux JF, Eckerbom P, Grenier N, Hall ME, Inoue T, Laustsen C, Lerman LO, Liu C, Morrell G, Pedersen M, Pruijm M, Sadowski EA, Seeliger E, Sharma K, Thoeny H, Vermathen P, Wang ZJ, Serafin Z, Zhang JL, Francis ST, Sourbron S, Pohlmann A, Fain SB, Prasad PV: Consensus-based technical recommendations for clinical translation of renal BOLD MRI. MAGMA 33: 199–215, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kainuma M, Yamada M, Miyake T: Continuous urine oxygen tension monitoring in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth 10: 603–608, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Lankadeva YR, Kosaka J, Evans RG, Bailey SR, Bellomo R, May CN: Intrarenal and urinary oxygenation during norepinephrine resuscitation in ovine septic acute kidney injury. Kidney Int 90: 100–108, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Zhu MZL, Martin A, Cochrane AD, Smith JA, Thrift AG, Harrop GK, Ngo JP, Evans RG: Urinary hypoxia: An intraoperative marker of risk of cardiac surgery-associated acute kidney injury. Nephrol Dial Transplant 33: 2191–2201, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Prasad PV, Priatna A, Spokes K, Epstein FH: Changes in intrarenal oxygenation as evaluated by BOLD MRI in a rat kidney model for radiocontrast nephropathy. J Magn Reson Imaging 13: 744–747, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu L, Tiwari MM, Messer KJ, Holthoff JH, Gokden N, Brock RW, Mayeux PR: Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol 292: F261–F268, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Post EH, Kellum JA, Bellomo R, Vincent JL: Renal perfusion in sepsis: From macro- to microcirculation. Kidney Int 91: 45–60, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Heyman SN, Brezis M, Epstein FH, Spokes K, Silva P, Rosen S: Early renal medullary hypoxic injury from radiocontrast and indomethacin. Kidney Int 40: 632–642, 1991 [DOI] [PubMed] [Google Scholar]
- 39.Briguori C, Donnarumma E, Quintavalle C, Fiore D, Condorelli G: Contrast-induced acute kidney injury: Potential new strategies. Curr Opin Nephrol Hypertens 24: 145–153, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Tanaka S, Tanaka T, Nangaku M: Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Renal Physiol 307: F1187–F1195, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Basile DP, Donohoe DL, Roethe K, Mattson DL: Chronic renal hypoxia after acute ischemic injury: Effects of L-arginine on hypoxia and secondary damage. Am J Physiol Renal Physiol 284: F338–F348, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR: Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol 294: F928–F936, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Polichnowski AJ, Lan R, Geng H, Griffin KA, Venkatachalam MA, Bidani AK: Severe renal mass reduction impairs recovery and promotes fibrosis after AKI. J Am Soc Nephrol 25: 1496–1507, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK: Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 26: 1765–1776, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nangaku M, Hirakawa Y, Mimura I, Inagi R, Tanaka T: Epigenetic changes in the acute kidney injury-to-chronic kidney disease transition. Nephron 137: 256–259, 2017 [DOI] [PubMed] [Google Scholar]
- 46.Mimura I, Tanaka T, Nangaku M: Novel therapeutic strategy with hypoxia-inducible factors via reversible epigenetic regulation mechanisms in progressive tubulointerstitial fibrosis. Semin Nephrol 33: 375–382, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Zager RA, Johnson AC: Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems and alters histone expression at proinflammatory/profibrotic genes. Am J Physiol Renal Physiol 296: F1032–F1041, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mimura I, Hirakawa Y, Kanki Y, Nakaki R, Suzuki Y, Tanaka T, Aburatani H, Nangaku M: Genome-wide analysis revealed that DZNep reduces tubulointerstitial fibrosis via down-regulation of pro-fibrotic genes. Sci Rep 8: 3779, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franzén S, Pihl L, Khan N, Gustafsson H, Palm F: Pronounced kidney hypoxia precedes albuminuria in type 1 diabetic mice. Am J Physiol Renal Physiol 310: F807–F809, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Welch WJ, Baumgärtl H, Lübbers D, Wilcox CS: Renal oxygenation defects in the spontaneously hypertensive rat: Role of AT1 receptors. Kidney Int 63: 202–208, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Pruijm M, Milani B, Pivin E, Podhajska A, Vogt B, Stuber M, Burnier M: Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int 93: 932–940, 2018 [DOI] [PubMed] [Google Scholar]
- 52.Sugiyama K, Inoue T, Kozawa E, Ishikawa M, Shimada A, Kobayashi N, Tanaka J, Okada H: Reduced oxygenation but not fibrosis defined by functional magnetic resonance imaging predicts the long-term progression of chronic kidney disease. Nephrol Dial Transplant 35: 964–970, 2020 [DOI] [PubMed] [Google Scholar]
- 53.Friederich-Persson M, Thörn E, Hansell P, Nangaku M, Levin M, Palm F: Kidney hypoxia, attributable to increased oxygen consumption, induces nephropathy independently of hyperglycemia and oxidative stress. Hypertension 62: 914–919, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rabinowitz MH: Inhibition of hypoxia-inducible factor prolyl hydroxylase domain oxygen sensors: Tricking the body into mounting orchestrated survival and repair responses. J Med Chem 56: 9369–9402, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr.: HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science 292: 464–468, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ: Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Rosenberger C, Mandriota S, Jürgensen JS, Wiesener MS, Hörstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU: Expression of hypoxia-inducible factor-1α and -2α in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13: 1721–1732, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Ratcliffe PJ: HIF-1 and HIF-2: Working alone or together in hypoxia? J Clin Invest 117: 862–865, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Semenza GL, Wang GL: A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12: 5447–5454, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schuster SJ, Badiavas EV, Costa-Giomi P, Weinmann R, Erslev AJ, Caro J: Stimulation of erythropoietin gene transcription during hypoxia and cobalt exposure. Blood 73: 13–16, 1989 [PubMed] [Google Scholar]
- 61.Koury ST, Koury MJ, Bondurant MC, Caro J, Graber SE: Quantitation of erythropoietin-producing cells in kidneys of mice by in situ hybridization: Correlation with hematocrit, renal erythropoietin mRNA, and serum erythropoietin concentration. Blood 74: 645–651, 1989 [PubMed] [Google Scholar]
- 62.Montero D, Lundby C: Arterial oxygen content regulates plasma erythropoietin independent of arterial oxygen tension: A blinded crossover study. Kidney Int 95: 173–177, 2019 [DOI] [PubMed] [Google Scholar]
- 63.Sugahara M, Tanaka T, Nangaku M: Prolyl hydroxylase domain inhibitors as a novel therapeutic approach against anemia in chronic kidney disease. Kidney Int 92: 306–312, 2017 [DOI] [PubMed] [Google Scholar]
- 64.Sanghani NS, Haase VH: Hypoxia-inducible factor activators in renal anemia: Current clinical experience. Adv Chronic Kidney Dis 26: 253–266, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee JW, Ko J, Ju C, Eltzschig HK: Hypoxia signaling in human diseases and therapeutic targets. Exp Mol Med 51: 1–13, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eckardt KU: The noblesse of kidney physiology. Kidney Int 96: 1250–1253, 2019 [DOI] [PubMed] [Google Scholar]
- 67.Matsumoto M, Makino Y, Tanaka T, Tanaka H, Ishizaka N, Noiri E, Fujita T, Nangaku M: Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol 14: 1825–1832, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Ma D, Lim T, Xu J, Tang H, Wan Y, Zhao H, Hossain M, Maxwell PH, Maze M: Xenon preconditioning protects against renal ischemic-reperfusion injury via HIF-1α activation. J Am Soc Nephrol 20: 713–720, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bernhardt WM, Câmpean V, Kany S, Jürgensen JS, Weidemann A, Warnecke C, Arend M, Klaus S, Günzler V, Amann K, Willam C, Wiesener MS, Eckardt KU: Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol 17: 1970–1978, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Iguchi M, Kakinuma Y, Kurabayashi A, Sato T, Shuin T, Hong SB, Schmidt LS, Furihata M: Acute inactivation of the VHL gene contributes to protective effects of ischemic preconditioning in the mouse kidney. Nephron, Exp Nephrol 110: e82–e90, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hill P, Shukla D, Tran MG, Aragones J, Cook HT, Carmeliet P, Maxwell PH: Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 19: 39–46, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kojima I, Tanaka T, Inagi R, Kato H, Yamashita T, Sakiyama A, Ohneda O, Takeda N, Sata M, Miyata T, Fujita T, Nangaku M: Protective role of hypoxia-inducible factor-2α against ischemic damage and oxidative stress in the kidney. J Am Soc Nephrol 18: 1218–1226, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Tanaka T, Kojima I, Ohse T, Inagi R, Miyata T, Ingelfinger JR, Fujita T, Nangaku M: Hypoxia-inducible factor modulates tubular cell survival in cisplatin nephrotoxicity. Am J Physiol Renal Physiol 289: F1123–F1133, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Bernhardt WM, Gottmann U, Doyon F, Buchholz B, Campean V, Schödel J, Reisenbuechler A, Klaus S, Arend M, Flippin L, Willam C, Wiesener MS, Yard B, Warnecke C, Eckardt KU: Donor treatment with a PHD-inhibitor activating HIFs prevents graft injury and prolongs survival in an allogenic kidney transplant model. Proc Natl Acad Sci U S A 106: 21276–21281, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ito M, Tanaka T, Ishii T, Wakashima T, Fukui K, Nangaku M: Prolyl hydroxylase inhibition protects the kidneys from ischemia via upregulation of glycogen storage. Kidney Int 97: 687–701, 2020 [DOI] [PubMed] [Google Scholar]
- 76.Kapitsinou PP, Jaffe J, Michael M, Swan CE, Duffy KJ, Erickson-Miller CL, Haase VH: Preischemic targeting of HIF prolyl hydroxylation inhibits fibrosis associated with acute kidney injury. Am J Physiol Renal Physiol 302: F1172–F1179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Z, Schley G, Türkoglu G, Burzlaff N, Amann KU, Willam C, Eckardt KU, Bernhardt WM: The protective effect of prolyl-hydroxylase inhibition against renal ischaemia requires application prior to ischaemia but is superior to EPO treatment. Nephrol Dial Transplant 27: 929–936, 2012 [DOI] [PubMed] [Google Scholar]
- 78.Rosenberger C, Khamaisi M, Abassi Z, Shilo V, Weksler-Zangen S, Goldfarb M, Shina A, Zibertrest F, Eckardt KU, Rosen S, Heyman SN: Adaptation to hypoxia in the diabetic rat kidney. Kidney Int 73: 34–42, 2008 [DOI] [PubMed] [Google Scholar]
- 79.Singh P, Blantz RC, Rosenberger C, Gabbai FB, Schoeb TR, Thomson SC: Aberrant tubuloglomerular feedback and HIF-1α confer resistance to ischemia after subtotal nephrectomy. J Am Soc Nephrol 23: 483–493, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanaka T, Nangaku M: Angiogenesis and hypoxia in the kidney. Nat Rev Nephrol 9: 211–222, 2013 [DOI] [PubMed] [Google Scholar]
- 81.Haase VH: Pathophysiological consequences of HIF activation: HIF as a modulator of fibrosis. Ann N Y Acad Sci 1177: 57–65, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, Kubo A, Akai Y, Rankin EB, Neilson EG, Haase VH, Saito Y: Stable expression of HIF-1α in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol 295: F1023–F1029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiang CK, Tanaka T, Inagi R, Fujita T, Nangaku M: Indoxyl sulfate, a representative uremic toxin, suppresses erythropoietin production in a HIF-dependent manner. Lab Invest 91: 1564–1571, 2011 [DOI] [PubMed] [Google Scholar]
- 84.Tanaka T, Yamaguchi J, Higashijima Y, Nangaku M: Indoxyl sulfate signals for rapid mRNA stabilization of Cbp/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 2 (CITED2) and suppresses the expression of hypoxia-inducible genes in experimental CKD and uremia. FASEB J 27: 4059–4075, 2013 [DOI] [PubMed] [Google Scholar]
- 85.Katavetin P, Miyata T, Inagi R, Tanaka T, Sassa R, Ingelfinger JR, Fujita T, Nangaku M: High glucose blunts vascular endothelial growth factor response to hypoxia via the oxidative stress-regulated hypoxia-inducible factor/hypoxia-responsible element pathway. J Am Soc Nephrol 17: 1405–1413, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Bento CF, Pereira P: Regulation of hypoxia-inducible factor 1 and the loss of the cellular response to hypoxia in diabetes. Diabetologia 54: 1946–1956, 2011 [DOI] [PubMed] [Google Scholar]
- 87.Tanaka T, Kojima I, Ohse T, Ingelfinger JR, Adler S, Fujita T, Nangaku M: Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest 85: 1292–1307, 2005 [DOI] [PubMed] [Google Scholar]
- 88.Tanaka T, Matsumoto M, Inagi R, Miyata T, Kojima I, Ohse T, Fujita T, Nangaku M: Induction of protective genes by cobalt ameliorates tubulointerstitial injury in the progressive Thy1 nephritis. Kidney Int 68: 2714–2725, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Nordquist L, Friederich-Persson M, Fasching A, Liss P, Shoji K, Nangaku M, Hansell P, Palm F: Activation of hypoxia-inducible factors prevents diabetic nephropathy. J Am Soc Nephrol 26: 328–338, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schley G, Klanke B, Kalucka J, Schatz V, Daniel C, Mayer M, Goppelt-Struebe M, Herrmann M, Thorsteinsdottir M, Palsson R, Beneke A, Katschinski DM, Burzlaff N, Eckardt KU, Weidemann A, Jantsch J, Willam C: Mononuclear phagocytes orchestrate prolyl hydroxylase inhibition-mediated renoprotection in chronic tubulointerstitial nephritis. Kidney Int 96: 378–396, 2019 [DOI] [PubMed] [Google Scholar]
- 91.Kobayashi H, Gilbert V, Liu Q, Kapitsinou PP, Unger TL, Rha J, Rivella S, Schlöndorff D, Haase VH: Myeloid cell-derived hypoxia-inducible factor attenuates inflammation in unilateral ureteral obstruction-induced kidney injury. J Immunol 188: 5106–5115, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH: Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117: 3810–3820, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Theilig F, Enke AK, Scolari B, Polzin D, Bachmann S, Koesters R: Tubular deficiency of von Hippel-Lindau attenuates renal disease progression in anti-GBM glomerulonephritis. Am J Pathol 179: 2177–2188, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Futatsugi K, Tokuyama H, Shibata S, Naitoh M, Kanda T, Minakuchi H, Yamaguchi S, Hayashi K, Minamishima YA, Yanagita M, Wakino S, Itoh H: Obesity-induced kidney injury is attenuated by amelioration of aberrant PHD2 activation in proximal tubules. Sci Rep 6: 36533, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu X, Fang Y, Liu H, Zhu J, Zou J, Xu X, Jiang S, Ding X: The balance of beneficial and deleterious effects of hypoxia-inducible factor activation by prolyl hydroxylase inhibitor in rat remnant kidney depends on the timing of administration. Nephrol Dial Transplant 27: 3110–3119, 2012 [DOI] [PubMed] [Google Scholar]
- 96.Uchida T, Rossignol F, Matthay MA, Mounier R, Couette S, Clottes E, Clerici C: Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression in lung epithelial cells: Implication of natural antisense HIF-1alpha. J Biol Chem 279: 14871–14878, 2004 [DOI] [PubMed] [Google Scholar]
- 97.Rahtu-Korpela L, Karsikas S, Hörkkö S, Blanco Sequeiros R, Lammentausta E, Mäkelä KA, Herzig KH, Walkinshaw G, Kivirikko KI, Myllyharju J, Serpi R, Koivunen P: HIF prolyl 4-hydroxylase-2 inhibition improves glucose and lipid metabolism and protects against obesity and metabolic dysfunction. Diabetes 63: 3324–3333, 2014 [DOI] [PubMed] [Google Scholar]
- 98.Sugahara M, Tanaka S, Tanaka T, Saito H, Ishimoto Y, Wakashima T, Ueda M, Fukui K, Shimizu A, Inagi R, Yamauchi T, Kadowaki T, Nangaku M: Prolyl hydroxylase domain inhibitor protects against metabolic disorders and associated kidney disease in obese type 2 diabetic mice. J Am Soc Nephrol 31: 560–577, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hasegawa S, Tanaka T, Saito T, Fukui K, Wakashima T, Susaki EA, Ueda HR, Nangaku M: The oral hypoxia-inducible factor prolyl hydroxylase inhibitor enarodustat counteracts alterations in renal energy metabolism in the early stages of diabetic kidney disease. Kidney Int 97: 934–950, 2020 [DOI] [PubMed] [Google Scholar]
- 100.Mokas S, Larivière R, Lamalice L, Gobeil S, Cornfield DN, Agharazii M, Richard DE: Hypoxia-inducible factor-1 plays a role in phosphate-induced vascular smooth muscle cell calcification. Kidney Int 90: 598–609, 2016 [DOI] [PubMed] [Google Scholar]
- 101.Kraus A, Peters DJM, Klanke B, Weidemann A, Willam C, Schley G, Kunzelmann K, Eckardt KU, Buchholz B: HIF-1α promotes cyst progression in a mouse model of autosomal dominant polycystic kidney disease. Kidney Int 94: 887–899, 2018 [DOI] [PubMed] [Google Scholar]
- 102.Kraus A, Grampp S, Goppelt-Struebe M, Schreiber R, Kunzelmann K, Peters DJ, Leipziger J, Schley G, Schödel J, Eckardt KU, Buchholz B: P2Y2R is a direct target of HIF-1α and mediates secretion-dependent cyst growth of renal cyst-forming epithelial cells. Purinergic Signal 12: 687–695, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Minamishima YA, Moslehi J, Padera RF, Bronson RT, Liao R, Kaelin WG Jr.: A feedback loop involving the Phd3 prolyl hydroxylase tunes the mammalian hypoxic response in vivo. Mol Cell Biol 29: 5729–5741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Besarab A, Provenzano R, Hertel J, Zabaneh R, Klaus SJ, Lee T, Leong R, Hemmerich S, Yu KH, Neff TB: Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant 30: 1665–1673, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]